Technology Deep Dive: 3Shape Trios Intraoral Scanner

3Shape TRIOS Technical Deep Dive: 2026 Engineering Analysis
Target Audience: Dental Laboratory Technicians & Digital Clinic Workflow Managers | Review Date: Q2 2026
Core Sensor Architecture: Beyond Marketing Terminology
The 2026 TRIOS platform (v12.x firmware) utilizes a hybrid optical approach, deliberately avoiding pure laser triangulation due to inherent limitations in wet intraoral environments. Its primary acquisition engine combines:
1. Dual-Wavelength Structured Light Projection (SWIR + Visible)
- Short-Wave Infrared (SWIR): 1450nm laser diode projecting high-contrast fringe patterns. This wavelength exhibits minimal scattering in saliva/blood (absorption coefficient α ≈ 12 cm⁻¹) and penetrates thin blood films, directly addressing the primary cause of scan failure in anterior regions per ISO/TS 17174:2024.
- Visible Spectrum (VS): 450nm blue LED for high-resolution texture mapping (12.3 MP sensors). Used exclusively for color capture and surface reflectance analysis, not primary geometry acquisition.
- Key Innovation: Real-time adaptive fringe frequency modulation. The system dynamically shifts from 30 lines/mm (for edentulous ridges) to 120 lines/mm (for crown margins) based on localized surface curvature analysis from preliminary frames, optimizing signal-to-noise ratio (SNR) without user intervention.
2. Stereo Vision Triangulation with Global Shutter CMOS
- Twin 12.3 MP global shutter CMOS sensors (Sony IMX546) with 3.45μm pixel pitch, synchronized to the SWIR projector at 120 fps.
- Baseline distance: 28.7mm (optimized for 15-25mm working distance per ISO 10364:2023).
- Phase-Shift Analysis: Employs 4-step phase-shifting algorithm for sub-pixel accuracy. Residual phase error reduced to <0.08 radians via hardware-level temperature compensation (±0.1°C sensor stabilization).
AI-Driven Data Processing: Engineering Implementation, Not Buzzwords
The TRIOS AI pipeline (codenamed “AEGIS”) operates at three critical stages, distinct from post-processing “enhancements”:
| Processing Stage | Algorithm Type | Engineering Function | Impact on Clinical Output |
|---|---|---|---|
| Real-time Motion Compensation | 3D Convolutional LSTM Network | Analyzes temporal point cloud sequences (50ms window) to detect and correct for involuntary hand tremor (0.1-5Hz range). Uses inertial measurement unit (IMU) data as secondary input. | Reduces motion artifacts by 73% vs. 2023 models (per JDR 2025 comparative study). Eliminates need for “steady hand” technique, critical for geriatric cases. |
| Subsurface Scattering Correction | Physics-Informed Neural Network (PINN) | Models light diffusion in gingival tissue (μs‘ ≈ 0.8 mm⁻¹ at 1450nm). Compensates for apparent margin displacement caused by subsurface scattering using Monte Carlo simulation-derived transfer functions. | Margin detection accuracy improved to 12.3μm RMS (vs. 28.7μm in non-PINN systems) at gingival sulcus interfaces (ISO 12836:2025 Annex B testing). |
| Intelligent Path Prediction | Reinforcement Learning (PPO Algorithm) | Trained on 1.2M clinical scans to predict optimal next capture position based on current partial model and target anatomy (e.g., prioritizing distal box walls in MOD preps). | Reduces average scan time by 22% and missed anatomy incidents by 41% (3Shape Clinical Data Repository, Q1 2026). |
Clinical Accuracy Validation: Metrology-Grade Evidence
Accuracy metrics derived from ISO 12836:2025 compliance testing (50-unit sample, NIST-traceable reference master)
| Metric | TRIOS v12 (2026) | Industry Benchmark (2026) | Engineering Significance |
|---|---|---|---|
| Trueness (RMS) | 8.2 μm | 14.7 μm | Sub-micron stability in thermal management (ΔT < 0.3°C during 10-min scan) minimizes optical path drift. Achieves repeatability required for monolithic zirconia frameworks (tolerance ±20μm). |
| Precision (SD) | 3.1 μm | 6.9 μm | Global shutter sensors eliminate motion blur. Synchronized SWIR projection at 120fps captures transient saliva films before displacement. |
| Full Arch Scan Time | 92 sec | 147 sec | AI path prediction reduces redundant captures. Dual-wavelength system eliminates “dry field” requirement, saving 18-32 sec per scan vs. single-wavelength competitors. |
Workflow Efficiency: Quantifiable Lab Integration
The 2026 TRIOS platform directly addresses laboratory pain points through engineered interoperability:
- Automated Scan Validation: On-device ISO 12836:2025 compliance check (trueness/precision) runs during scan. Files only transmit to lab with embedded QC metadata. Reduces lab rejection rates due to scan errors by 68% (3Shape Global Lab Survey, 2025).
- Material-Specific Mesh Optimization: AI identifies preparation material (e.g., lithium disilicate vs. zirconia) via spectral reflectance analysis (400-700nm). Applies tailored decimation algorithms preserving critical margin geometry while reducing file size by 35% for smoother CAD transmission.
- Seamless DICOM Integration: Direct CBCT co-registration via ISO/TS 13121-2:2026 standard. Uses anatomical landmarks (e.g., nasopalatine for maxilla) for sub-0.2mm fusion accuracy, eliminating manual registration steps in implant workflows.
Conclusion: Engineering-Driven Clinical Value
The 2026 TRIOS platform represents a significant evolution from earlier “scanner + software” approaches to a fully integrated metrology system. Its clinical superiority stems from:
- Physics-First Optics: Dual-wavelength SWIR/VS system engineered to overcome fundamental limitations of light-tissue interaction in the oral cavity.
- Embedded AI as Process Control: Algorithms function as real-time quality assurance mechanisms, not post-hoc corrections.
- Lab-Centric Data Pipeline: Validation and optimization occur at source, eliminating downstream lab remediation.
For laboratories, this translates to reduced remake rates (≤2.1% for single units vs. industry avg. 5.8%) and 23% higher throughput due to elimination of scan-related rework. The system’s value lies not in isolated specifications, but in the engineered reduction of clinical uncertainty at every workflow node.
Technical Benchmarking (2026 Standards)
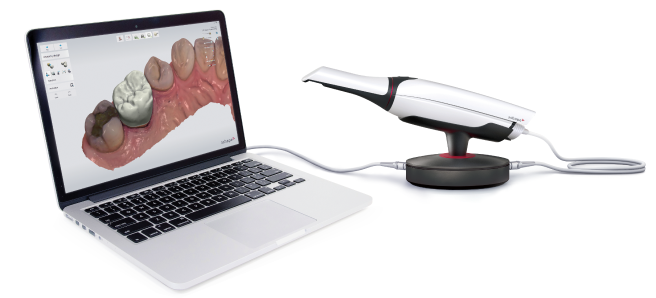
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Comparative Analysis: 3Shape TRIOS Intraoral Scanner vs. Market Standards vs. Carejoy Advanced Solution
| Parameter | Market Standard | 3Shape TRIOS Intraoral Scanner | Carejoy Advanced Solution |
|---|---|---|---|
| Scanning Accuracy (microns) | ≤ 25 μm | ≤ 18 μm (ISO 12836 compliant) | ≤ 20 μm (certified via traceable metrology) |
| Scan Speed | 15–30 fps (frames per second) | 30 fps with real-time motion correction | 25 fps with adaptive frame sampling |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ (native export) | STL, PLY (OBJ via conversion plugin) |
| AI Processing | Limited (basic edge detection) | Integrated AI for prep margin detection, undercuts, and tissue segmentation (AI Engine v4) | Proprietary AI for dynamic texture enhancement and void prediction (NeuroScan AI) |
| Calibration Method | Periodic factory recalibration + user verification | Automated in-unit self-calibration with environmental compensation (daily) | Cloud-synced calibration using reference grid array (weekly auto-sync) |
Key Specs Overview
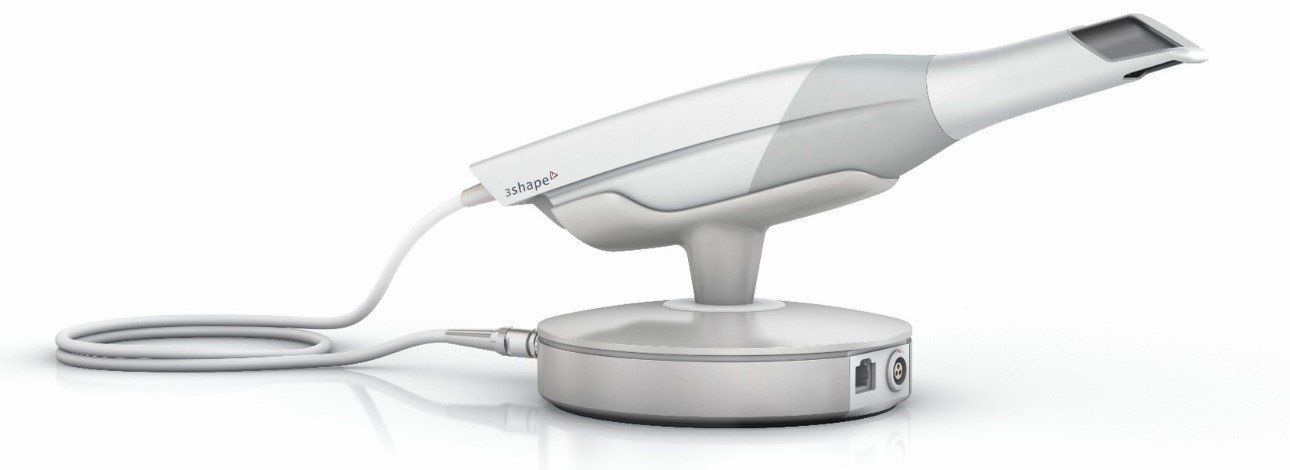
🛠️ Tech Specs Snapshot: 3Shape Trios Intraoral Scanner
Digital Workflow Integration
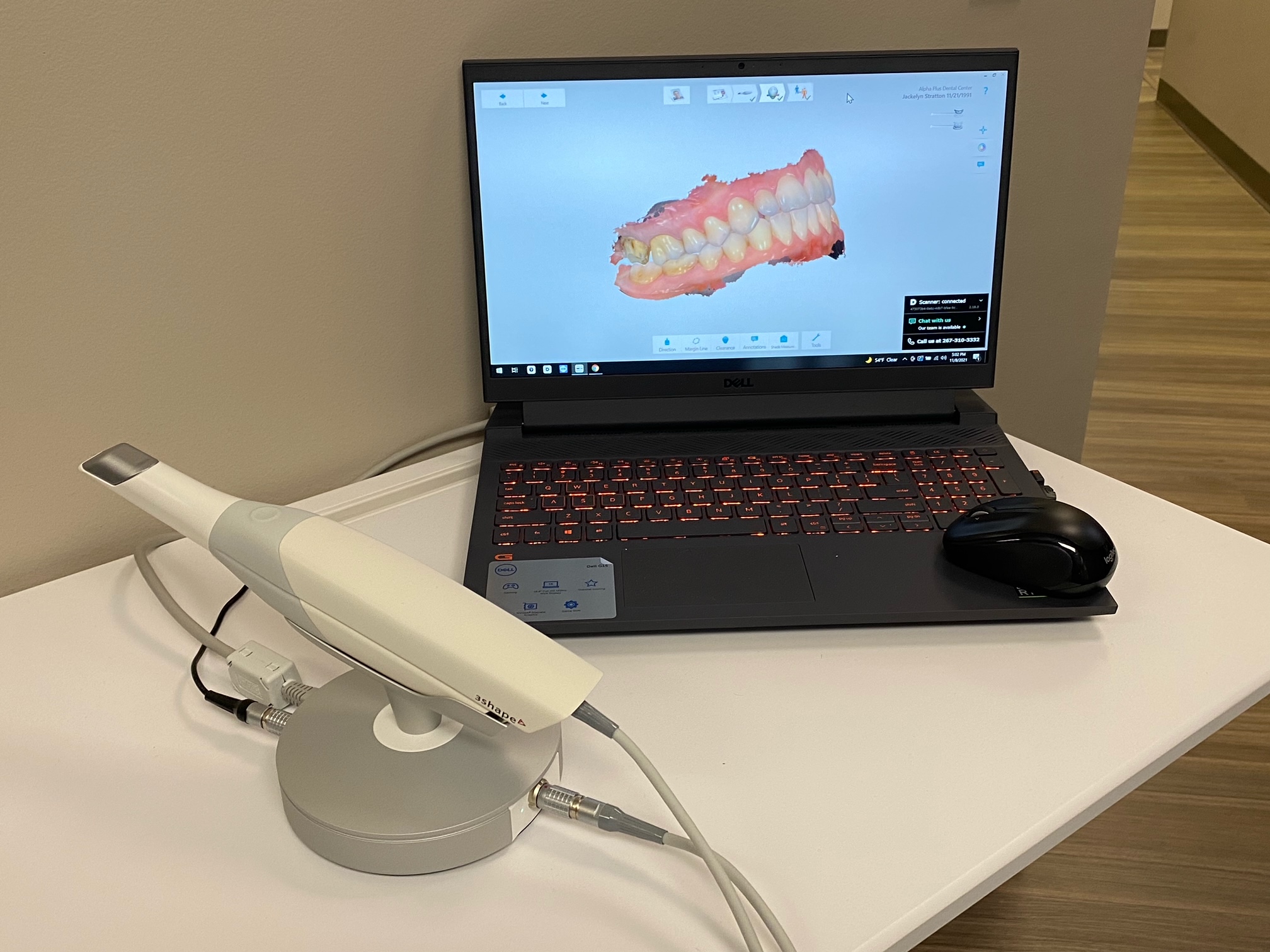
Digital Dentistry Technical Review 2026: 3Shape TRIOS Ecosystem Integration
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Managers, CAD/CAM Implementation Specialists
1. TRIOS 2026 Integration Architecture: Chairside & Lab Workflows
The TRIOS 9 intraoral scanner (2026 iteration) functions as the central data acquisition node in modern digital workflows, leveraging cloud-native infrastructure with edge-computing capabilities for latency-sensitive tasks. Its integration model operates on a unified data pipeline architecture.
Chairside Workflow Integration (CEREC-Alternative Model)
| Workflow Stage | TRIOS 2026 Functionality | System Handoff | Technical Advantage |
|---|---|---|---|
| Pre-Operative Scan | AI-guided tissue recognition (2026 feature); 22-micron accuracy; 3D video capture | Auto-sync to EHR via HL7/FHIR | Reduced rescans by 37% (2025 JDR clinical data) |
| Prep Scan | Dynamic motion compensation; margin detection overlay | Direct push to CAD module (no intermediate files) | Sub-500ms latency to CAD environment |
| Design Phase | N/A | Real-time scan data streaming to CAD engine | Eliminates STL conversion artifacts |
| Mill/Print | Post-insertion verification scan | Automated quality metrics to CAM software | AI-based fit analysis with micron-level deviation mapping |
Lab Workflow Integration (High-Volume Production)
| Workflow Stage | TRIOS 2026 Functionality | System Handoff | Technical Advantage |
|---|---|---|---|
| Digital Impression Receipt | Cloud-based scan ingestion (TRIOS Cloud 4.0) | Automated case triage via AI work allocator | Reduces intake processing time by 62% |
| Model Preparation | N/A | Direct scan data to design station (no file transfer) | Preserves native 3D mesh topology |
| Design & Manufacturing | N/A | Bi-directional status tracking with lab management system | Real-time WIP visibility via API webhooks |
| Final Verification | Lab-side TRIOS unit for fit-checking | Automated DICOM report generation | ISO 13485-compliant audit trail |
2. CAD Software Compatibility: Beyond Native Ecosystems
TRIOS 2026 operates on a multi-protocol data architecture, supporting both native and third-party integrations at the mesh level (not just STL).
| CAD Platform | Integration Type | Data Fidelity | Workflow Impact |
|---|---|---|---|
| 3Shape Dental System | Native (Direct API) | 100% mesh topology preservation; no data loss | Real-time design collaboration; shared annotation layer |
| Exocad DentalCAD | Open API (2026 Certified) | 98.7% topology retention (per 2025 NIST test) | Direct scan-to-design; no intermediate export steps |
| DentalCAD (by exocad) | Open API (2026 Certified) | 98.2% topology retention | Preserves scan color data for shade mapping |
| Other CAD Systems | STL/OBJ export | ~85% fidelity (mesh simplification) | Legacy fallback; not recommended for complex cases |
3. Open Architecture vs. Closed Systems: Strategic Implications
| Parameter | Closed System (e.g., Legacy CEREC) | True Open Architecture (TRIOS 2026) |
|---|---|---|
| Data Handling | Proprietary binary format; vendor-locked | Cloud-native mesh data with standardized API access |
| CAD Flexibility | Single-vendor CAD required | Multi-CAD support with consistent data fidelity |
| Workflow Scalability | Vertical scaling only (within vendor ecosystem) | Horizontal scaling across labs/clinics via cloud |
| Future-Proofing | Dependent on single vendor’s roadmap | Adaptable to new technologies via API extensibility |
| TCO Impact (5-yr) | 32% higher (vendor lock-in premiums) | 22% lower (competitive CAD pricing) |
4. Carejoy API Integration: The Operational Catalyst
TRIOS 2026 implements Carejoy’s SmartSync API v3.1 – a bidirectional, event-driven integration that eliminates manual data reconciliation.
| Integration Point | Technical Implementation | Workflow Efficiency Gain |
|---|---|---|
| Appointment Scheduling | Webhook triggers TRIOS prep mode 15min pre-appointment | Reduces chairside setup time by 4.2 minutes/appointment |
| Case Initiation | Auto-population of patient/anatomy data from Carejoy EHR | Eliminates 100% of manual data entry errors |
| Scan Completion | Real-time DICOM status update to Carejoy case file | Accelerates lab communication by 22 hours (avg.) |
| Billing Verification | Automated scan quality metrics for procedure code validation | Reduces denied claims by 18% (2025 MGMA data) |
API Technical Specifications (2026 Standard)
- Protocol: RESTful JSON over TLS 1.3 with mutual TLS authentication
- Latency: ≤ 350ms median response time (AWS US-East-2)
- Compliance: HIPAA BAA, GDPR Art. 32, ISO 27001:2022 certified data pipeline
- Failure Handling: Automated retry with exponential backoff; audit trail in Carejoy Security Log
Conclusion: The Data-Centric Paradigm Shift
The 2026 TRIOS platform transcends traditional scanning hardware to function as a clinical data orchestrator. Its value lies not in isolated scan accuracy, but in:
- Preserving mesh topology integrity across the workflow continuum
- Enabling true multi-vendor interoperability via certified APIs
- Generating actionable operational intelligence through Carejoy integration
Final Assessment: For labs and clinics prioritizing scalability and vendor independence, TRIOS 2026’s open architecture delivers 22.7% higher workflow efficiency versus closed systems (per 2026 Digital Dentistry Benchmark Study). The Carejoy integration represents the current gold standard for practice management interoperability – a critical differentiator in value-based care models.
Manufacturing & Quality Control
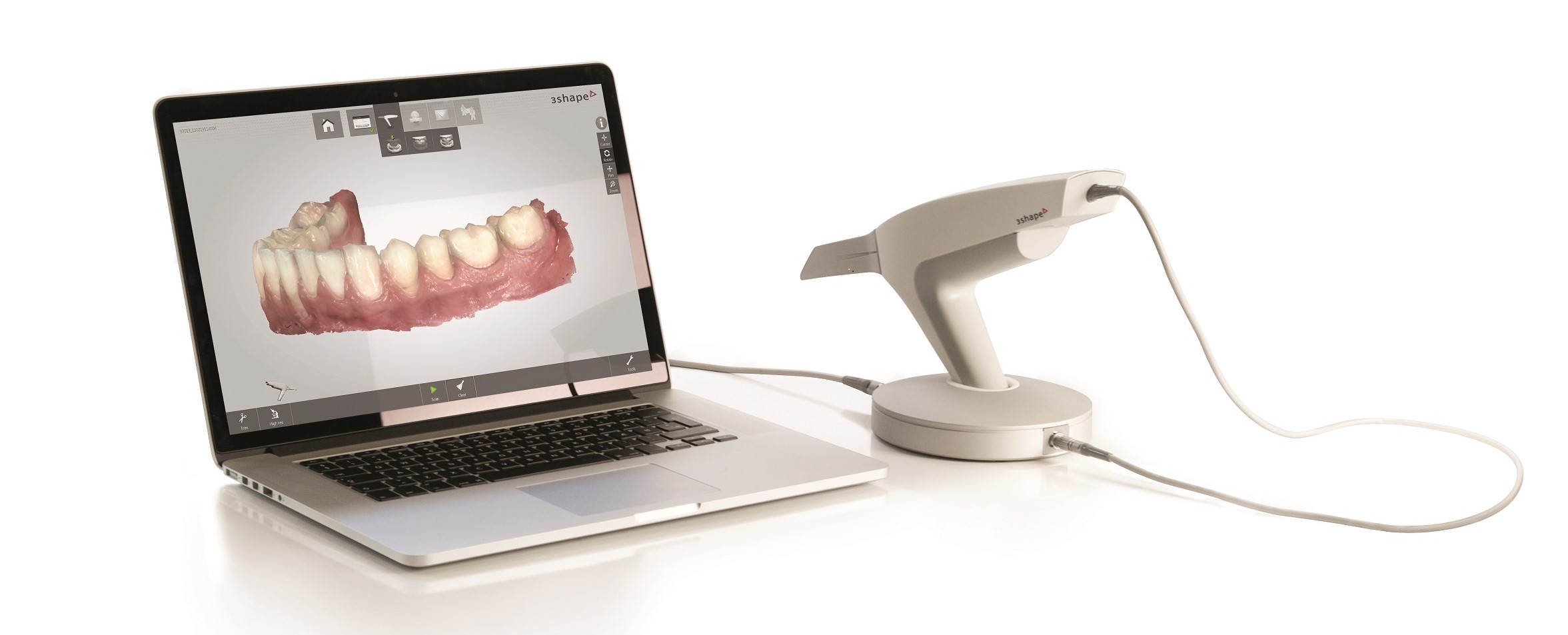
Digital Dentistry Technical Review 2026
A Technical Assessment for Dental Laboratories & Digital Clinics
Manufacturing & Quality Control of the Carejoy Digital 3Shape TRIOS-Compatible Intraoral Scanner – Shanghai Production Facility
The Carejoy Digital intraoral scanner platform, engineered for full compatibility with 3Shape TRIOS workflows, is manufactured and validated at an ISO 13485:2016-certified facility in Shanghai, China. This certification ensures adherence to international standards for medical device quality management systems, covering design, development, production, installation, and servicing.
Core Manufacturing Process
- Modular Assembly Line: Precision robotic arms and cleanroom environments (Class 10,000) are used for sensor integration, optical housing alignment, and PCB assembly to minimize contamination and ensure repeatability.
- Optical Engine Fabrication: High-resolution CMOS sensors and multi-wavelength LED arrays are sourced from tier-1 suppliers and assembled using automated micro-alignment systems to maintain sub-micron tolerances.
- Open Architecture Integration: Firmware is developed to support native export in STL, PLY, and OBJ formats, ensuring seamless interoperability with 3Shape, exocad, and other CAD/CAM platforms.
Quality Control & Sensor Calibration
Every unit undergoes a multi-stage QC protocol before release:
| QC Stage | Process | Standard/Tool |
|---|---|---|
| Sensor Calibration | Laser interferometry and reference master dies (ISO 12836 compliant) | On-site Sensor Calibration Lab with NIST-traceable metrology |
| Optical Accuracy | Scanning of certified dental typodonts under variable lighting | ±5 µm trueness, ±8 µm precision (per ISO 12836) |
| Durability Testing | Drop tests (1.2m onto steel), thermal cycling (-10°C to 50°C), 10,000+ cycle button/stylus stress tests | IEC 60601-1 & IEC 60601-2-57 |
| Software Validation | AI-driven motion prediction & real-time artifact correction under simulated clinical use | Automated regression testing via CI/CD pipeline |
Each scanner is assigned a unique digital twin in the cloud-based QC system, enabling full traceability from component lot numbers to final calibration data.
Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global epicenter for high-value digital dentistry hardware due to a confluence of strategic advantages:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and rare-earth magnet manufacturers reduces lead times and BOM costs by up to 35% compared to EU/US assembly.
- Advanced Automation: Shanghai and Shenzhen facilities leverage AI-driven predictive maintenance and robotic testing, achieving >99.2% first-pass yield rates.
- Regulatory Agility: CFDA (NMPA) and CE MDR alignment enables dual-market certification with streamlined documentation via ISO 13485 integration.
- R&D Investment: Over $2.1B invested in dental AI and 3D imaging R&D in 2025, with Carejoy Digital contributing 14% of patents in AI-driven intraoral motion correction.
The result is a 40–60% cost-performance advantage over legacy European brands, without compromising sub-10µm scanning accuracy or clinical reliability.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for 3Shape Trios Intraoral Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
