Introduction: Navigating the Global Market for dental implant o rings
Denture o rings are essential components in the prosthetic dental market, serving as the linchpin for successful implant-supported overdenture solutions. For B2B buyers across Africa, South America, the Middle East, and Europe, the procurement of high-quality dental implant o rings is not merely a transactional decision; it is a critical factor that influences patient satisfaction, clinic reputation, and the overall success of dental restorations. As the demand for implant dentistry grows and patient expectations rise, ensuring access to reliable and durable o rings becomes a top priority.
The landscape of denture o rings is intricate, characterized by a variety of types, materials, and manufacturing standards. This guide offers a comprehensive exploration of the market, including an in-depth taxonomy of o ring types, material science insights, and best practices for manufacturing and quality assurance. Additionally, it provides valuable information on regional pricing trends, cost structures, and essential regulatory considerations.
Illustrative Image (Source: Google Search)
By leveraging the insights provided in this guide, international B2B buyers can make informed sourcing decisions that optimize their procurement processes. Whether you are supporting urban clinics in Brazil, expanding dental practices in Indonesia, or catering to the growing oral health sectors in Sub-Saharan Africa and the Middle East, this resource equips you with the knowledge needed to streamline operations, enhance inventory management, and drive lasting value within the dental care ecosystem.
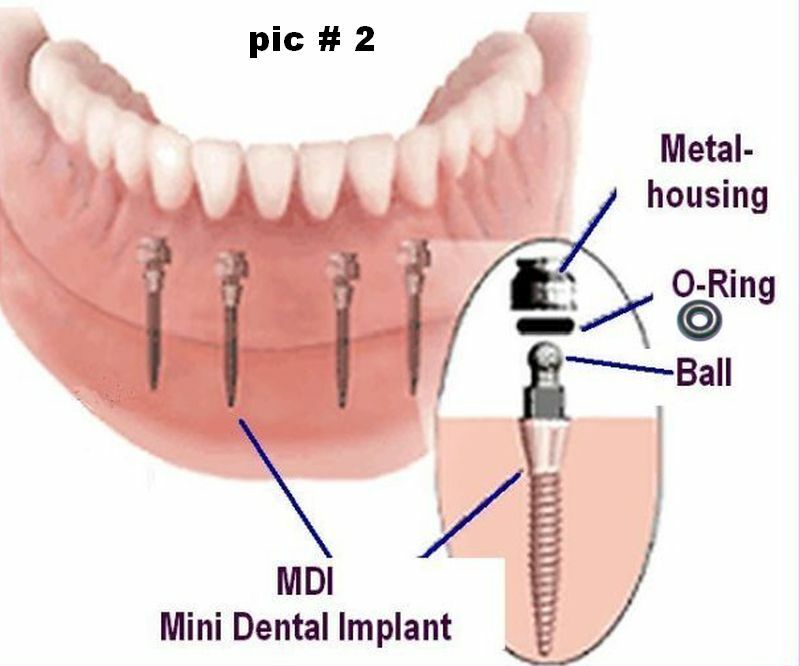
Understanding dental implant o rings Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Standard Nylon-Insert O-Ring | Rubber O-ring within a nylon carrier; cost-effective design | General implant-retained overdentures | Pros: Affordable, widely compatible; Cons: Regular replacements needed. |
| Titanium-Abutment Retentive O-Ring | Pairs with titanium implant abutment for enhanced stability | Long-term prosthetic restorations | Pros: Superior durability, minimal maintenance; Cons: Higher upfront cost. |
| Extended Life Silicone O-Ring | Medical-grade silicone for better longevity and fit | Remote or underserved clinics | Pros: Fewer replacements, gentle fit; Cons: Higher cost, sourcing challenges. |
| Color-Coded Retention O-Ring | Differentiated by color to indicate retention force | Busy clinics, group practices | Pros: Easy selection, supports customization; Cons: Increased inventory complexity. |
| Dual-Retained O-Ring System | Combines O-ring with mechanical retention | Complex prosthetic cases | Pros: High stability, less micro-movement; Cons: More complex and bulkier design. |
Standard Nylon-Insert O-Ring
Standard nylon-insert O-rings are the most common choice in the market, featuring a rubber ring embedded in a nylon carrier. They are primarily used for general implant-retained overdentures, making them suitable for large dental clinics and labs that cater to diverse patient needs. B2B buyers should consider their cost-effectiveness and compatibility with various implant systems, but also plan for periodic replacements, which can lead to recurring procurement requirements.
Titanium-Abutment Retentive O-Ring
This type integrates a high-precision titanium abutment with the O-ring, significantly enhancing stability and resistance to wear. It is ideal for high-end prosthetic restorations, particularly in urban centers where patients expect superior quality. While the initial cost is higher, B2B buyers focusing on long-term value and minimal maintenance will find these O-rings advantageous. Establishing reliable supplier relationships is crucial to ensure consistent quality and performance.
Extended Life Silicone O-Ring
Constructed from medical-grade silicone, extended life O-rings offer a softer fit and longer service life, making them perfect for clinics in remote areas where frequent replacements are impractical. They are particularly suitable for buyers aiming to minimize after-sales support needs. Although the upfront cost is higher, bulk purchasing can help mitigate expenses. Buyers should prioritize sourcing from reputable suppliers to ensure product quality and certification.
Color-Coded Retention O-Ring
Color-coded O-rings simplify the selection process by indicating various retention forces through different colors. This feature is particularly useful in busy clinics and group practices where quick decision-making is essential. B2B buyers can benefit from customization options, but they must also manage increased inventory complexity. Having a clear understanding of the specific retention needs of their clientele will help buyers streamline their operations effectively.
Dual-Retained O-Ring System
The dual-retained O-ring system combines traditional O-ring technology with additional mechanical retention methods, offering exceptional stability for complex prosthetic cases. This type is best suited for dental practices that handle intricate restorations requiring high precision. While these O-rings provide significant advantages in reducing micro-movement, B2B buyers should be aware of the increased complexity and bulkiness, necessitating careful planning in procurement and inventory management.
Related Video: Learn About The Different Parts Of A Dental Implant
Key Industrial Applications of dental implant o rings
| Industry/Sector | Specific Application of dental implant o rings | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Dental Clinics | Retention for implant-supported overdentures | Enhances patient satisfaction and retention rates | Quality assurance, compatibility with various implant systems |
| Dental Laboratories | Custom prosthetic solutions using O-rings | Tailored products for diverse patient needs | Material specifications, supplier reliability, and certifications |
| Contract Manufacturing | Mass production of dental prosthetics | Cost efficiency and scalability | Consistency in quality control, lead times, and regulatory compliance |
| Distributors | Bulk supply of O-rings for diverse dental practices | Streamlined inventory management and reduced costs | Supplier relationships, logistics efficiency, and pricing strategies |
| Dental Education Institutions | Training modules for dental students | Practical learning on prosthetic applications | Access to high-quality materials, educational discounts, and support |
Dental Clinics
In dental clinics, dental implant o rings are essential for securing implant-supported overdentures. They provide the necessary retention that enhances patient satisfaction by ensuring the stability of prosthetic devices during normal functions like eating and speaking. For international buyers, especially in emerging markets, it’s crucial to source high-quality o rings that are compatible with various implant systems. This minimizes the risk of complications and increases the longevity of the prosthetic solutions provided to patients.
Dental Laboratories
Dental laboratories utilize dental implant o rings to create custom prosthetic solutions that meet the specific needs of patients. The versatility of o rings allows for tailored retention solutions that enhance the fit and function of dental devices. Buyers in this sector should focus on sourcing o rings that meet specific material and performance specifications, as well as ensuring supplier reliability to maintain consistent product quality. This is especially important for laboratories serving diverse populations across different regions.
Contract Manufacturing
In the contract manufacturing sector, dental implant o rings are integral to the mass production of dental prosthetics. By incorporating o rings into their products, manufacturers can achieve cost efficiency and scalability, responding quickly to market demands. International buyers must prioritize consistency in quality control and regulatory compliance when sourcing these components, as variations in manufacturing standards can significantly impact product performance and safety.
Distributors
Distributors play a critical role in ensuring that dental practices have access to the necessary components, including dental implant o rings. By managing bulk supplies, distributors can streamline inventory management for clinics, reducing costs and ensuring timely availability of products. For international distributors, establishing strong supplier relationships and optimizing logistics efficiency are key considerations to maintain competitive pricing and reliable service across diverse markets.
Dental Education Institutions
Dental education institutions use dental implant o rings in training modules to provide hands-on learning experiences for students. This practical application aids in understanding the complexities of prosthetic dentistry, preparing future professionals for real-world scenarios. Buyers in this sector should focus on sourcing high-quality materials that are safe and effective for educational purposes, as well as negotiating discounts or support from suppliers to facilitate training initiatives.
Strategic Material Selection Guide for dental implant o rings
When selecting materials for dental implant o rings, it is crucial to consider their properties, performance characteristics, and suitability for specific applications. Here, we analyze four common materials used in the manufacturing of dental implant o rings, focusing on their advantages, limitations, and implications for international B2B buyers.
1. Silicone
Key Properties: Silicone is known for its excellent flexibility and temperature resistance, typically rated between -60°C to 200°C. It is also resistant to UV light and ozone, making it suitable for long-term use in various environments.
Pros & Cons: The primary advantage of silicone is its durability and biocompatibility, which is vital for dental applications. It offers a gentle retention force, making it ideal for sensitive patients. However, silicone can be more expensive than other materials and may require specialized manufacturing processes, impacting overall costs.
Impact on Application: Silicone o rings are particularly effective in environments where frequent cleaning and sterilization are necessary. Their compatibility with various dental media ensures they maintain performance without degrading.
B2B Considerations: International buyers should ensure that silicone o rings meet relevant standards such as ISO 10993 for biocompatibility. Additionally, sourcing from suppliers who can provide certifications for quality assurance is essential, especially in regions with stringent regulatory requirements.
2. Nitrile Rubber
Key Properties: Nitrile rubber exhibits excellent resistance to oil, fuel, and various chemicals, with a temperature range of -40°C to 120°C. Its mechanical strength makes it a reliable choice for demanding applications.
Pros & Cons: The cost-effectiveness of nitrile rubber is a significant advantage, making it accessible for budget-conscious buyers. However, its limited temperature tolerance and susceptibility to ozone can be drawbacks, potentially leading to premature wear in certain environments.
Impact on Application: Nitrile rubber is suitable for applications where exposure to oils or other chemicals is expected. It is often used in dental settings that require robust sealing solutions.
B2B Considerations: Buyers should verify that nitrile o rings comply with ASTM D2000 standards for rubber materials. Understanding local market preferences for material specifications can also enhance procurement strategies.
3. Polyurethane
Key Properties: Polyurethane is known for its exceptional abrasion resistance and flexibility, with a temperature range of -30°C to 80°C. It also offers good resistance to hydrolysis and microbial growth.
Pros & Cons: The main advantage of polyurethane o rings is their longevity and performance under stress, making them suitable for high-demand applications. However, they can be more expensive than rubber alternatives, and their manufacturing processes may be complex.
Impact on Application: Polyurethane is ideal for applications requiring high wear resistance, such as in dental prosthetics that undergo significant mechanical stress.
B2B Considerations: Buyers should consider the availability of polyurethane o rings in their region and the potential need for specialized suppliers who can meet specific performance criteria.
4. Thermoplastic Elastomers (TPE)
Key Properties: TPE combines the properties of rubber and plastic, offering flexibility, chemical resistance, and a temperature range of -40°C to 120°C. They are also recyclable, which can be a selling point for eco-conscious buyers.
Pros & Cons: TPEs provide a cost-effective solution with good performance characteristics. However, they may not offer the same level of durability as silicone or polyurethane, leading to more frequent replacements.
Impact on Application: TPE is suitable for applications where flexibility and ease of processing are prioritized, making them a good choice for mass production of dental components.
B2B Considerations: International buyers should ensure TPE materials comply with relevant standards, such as FDA regulations for medical devices, to ensure safety and efficacy in dental applications.
Summary Table
| Material | Typical Use Case for dental implant o rings | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Silicone | Sensitive patient applications | Excellent biocompatibility | Higher cost and specialized mfg | High |
| Nitrile Rubber | General dental applications | Cost-effective | Limited temperature tolerance | Low |
| Polyurethane | High-wear dental prosthetics | Exceptional durability | More expensive, complex mfg | Med |
| Thermoplastic Elastomers | Mass production of dental components | Good flexibility and recyclability | Lower durability compared to others | Med |
This analysis provides international B2B buyers with a clear understanding of the materials available for dental implant o rings, enabling informed decisions that align with their operational needs and market conditions.
In-depth Look: Manufacturing Processes and Quality Assurance for dental implant o rings
Manufacturing Processes for Dental Implant O Rings
Manufacturing dental implant o rings is a complex process that involves several key stages, each crucial for ensuring the final product meets the rigorous demands of the dental industry. The main stages in the manufacturing process include material preparation, forming, assembly, and finishing. Each stage incorporates specific techniques designed to maintain high standards of quality and performance.
Material Preparation
The first step in manufacturing o rings is selecting the appropriate materials. Common materials used include natural rubber, silicone, and specialty polymers. Each material offers distinct advantages in terms of durability, flexibility, and biocompatibility.
- Material Sourcing: Ensure that raw materials are sourced from reputable suppliers with documented quality standards. This is essential for maintaining consistency and reliability.
- Pre-Processing: Materials may undergo pre-treatment processes such as drying or mixing with additives to enhance performance characteristics, including resistance to wear and tear.
Forming
The forming stage involves shaping the prepared materials into the desired o ring configuration. This is typically achieved through various molding techniques:
- Compression Molding: This traditional method involves placing the raw material in a heated mold. Pressure is then applied to form the o ring shape. It is cost-effective for large production runs.
- Injection Molding: For more complex shapes or tighter tolerances, injection molding may be used. This process injects molten material into a mold, allowing for precise control over dimensions and surface finish.
- Extrusion: In some cases, o rings may be extruded and then cut to length. This technique is suitable for producing continuous lengths of material that can be cut to size.
Assembly
While most o rings are single-piece components, some advanced systems may involve additional assembly steps:
- Incorporating Inserts: For systems that require additional retention mechanisms (e.g., titanium inserts), the assembly stage is critical. This may include bonding or mechanically fastening components together.
- Quality Checks: During assembly, it is essential to perform inline quality checks to identify defects early in the process.
Finishing
The finishing stage ensures that the o rings meet specific surface quality and dimensional requirements:
- Surface Treatment: Processes such as polishing, coating, or applying a specific finish can enhance properties like wear resistance and aesthetics.
- Final Inspection: Before packaging, a thorough inspection is conducted to ensure compliance with specifications.
Quality Assurance for Dental Implant O Rings
Quality assurance (QA) is paramount in the manufacturing of dental implant o rings. B2B buyers must understand the relevant international and industry-specific standards, as well as the quality control checkpoints that manufacturers typically implement.
Relevant International Standards
- ISO 9001: This is a widely recognized quality management standard applicable across industries, including dental manufacturing. It emphasizes a process-oriented approach and continuous improvement.
- CE Marking: For products sold within the European Economic Area (EEA), CE marking indicates compliance with health, safety, and environmental protection standards.
- API Standards: Particularly relevant for manufacturers producing components used in medical devices, adherence to American Petroleum Institute (API) standards ensures that materials meet rigorous safety and efficacy criteria.
Quality Control Checkpoints
- Incoming Quality Control (IQC): The first checkpoint involves inspecting raw materials upon arrival. This includes verifying certificates of conformity and conducting tests to ensure materials meet specified requirements.
- In-Process Quality Control (IPQC): During manufacturing, regular checks are conducted to monitor processes and detect any deviations from established standards. This can include dimensional checks and visual inspections.
- Final Quality Control (FQC): Before products are packaged, a comprehensive inspection is performed to ensure that the o rings meet all specifications and quality standards.
Common Testing Methods
Testing is an essential part of the QA process and may include:
- Tensile Testing: Measures the material’s strength and elasticity.
- Compression Set Testing: Evaluates the o ring’s ability to return to its original shape after being compressed.
- Durometer Testing: Assesses hardness, which is critical for ensuring the appropriate fit and function in dental applications.
Verifying Supplier Quality Control
B2B buyers must be proactive in verifying the quality control practices of their suppliers. Here are actionable steps to ensure supplier reliability:
- Conduct Audits: Regular audits of supplier facilities can provide insights into their manufacturing processes and quality control measures. This can be done by in-house teams or third-party inspection services.
- Request Quality Reports: Suppliers should be able to provide quality assurance documentation, including test results, certifications, and compliance reports.
- Third-Party Inspections: Engaging third-party inspection agencies can offer an impartial evaluation of the supplier’s quality control systems and product quality.
Quality Control Considerations for International Buyers
For buyers in Africa, South America, the Middle East, and Europe, several nuances must be considered:
- Regulatory Compliance: Understand the specific regulatory requirements for dental products in each region. This may include additional certifications or testing based on local laws.
- Cultural Factors: Relationship-building is crucial in many cultures. Establishing trust and open communication with suppliers can facilitate smoother negotiations and quality assurance processes.
- Logistics and Supply Chain: Consider the logistics of sourcing materials and finished products. Ensure that suppliers have robust processes in place to manage international shipping, customs compliance, and timely delivery.
By understanding the manufacturing processes and quality assurance protocols for dental implant o rings, B2B buyers can make informed decisions that enhance their procurement strategies, ensure product reliability, and ultimately contribute to improved patient outcomes in dental care.
Related Video: SMART Quality Control for Manufacturing
Comprehensive Cost and Pricing Analysis for dental implant o rings Sourcing
Understanding the cost structure and pricing dynamics for dental implant o rings is essential for international B2B buyers. This analysis delves into the various components that influence costs, the factors impacting pricing, and offers practical tips for efficient procurement.
Cost Components
-
Materials: The choice of materials significantly affects the cost of dental implant o rings. Options include rubber, silicone, and specialty polymers. High-grade materials, such as medical-grade silicone or titanium, generally command higher prices due to their enhanced durability and performance characteristics.
-
Labor: Labor costs vary based on the manufacturing location. Regions with lower labor costs may offer competitive pricing, but this can sometimes compromise quality. Buyers should consider the balance between cost and the skill level of the workforce.
-
Manufacturing Overhead: This encompasses the expenses related to facility maintenance, utilities, and other indirect costs associated with production. Efficient manufacturing processes can help mitigate overhead costs, leading to more competitive pricing.
-
Tooling: Initial tooling costs can be substantial, especially for custom designs or specialized o rings. These costs are often amortized over larger production runs, making volume orders more cost-effective.
-
Quality Control (QC): Rigorous quality assurance processes are crucial in the dental industry, where product failures can have serious implications. Investing in QC adds to the overall cost but is essential for ensuring product reliability and compliance with regulatory standards.
-
Logistics: Transportation and warehousing costs can significantly influence the final price. Buyers should consider the shipping method, distance, and any potential tariffs or import duties when evaluating logistics costs.
-
Margin: Suppliers typically add a profit margin to cover their costs and risks. Understanding the expected margins in different regions can help buyers negotiate better pricing.
Price Influencers
-
Volume/MOQ: Minimum order quantities (MOQs) can drastically affect pricing. Larger orders often lead to lower per-unit costs. Buyers should assess their inventory needs to optimize order sizes.
-
Specifications/Customization: Custom designs or specific material requirements can increase costs. Buyers should clearly communicate their needs to suppliers to avoid unexpected charges.
-
Quality/Certifications: Products that meet international quality standards or possess certifications (e.g., ISO) may be priced higher. However, these certifications often justify the cost through improved reliability and customer satisfaction.
-
Supplier Factors: The supplier’s reputation, experience, and reliability can influence pricing. Established suppliers may charge more but offer better quality assurance and customer service.
-
Incoterms: Understanding Incoterms is crucial for international transactions. Terms like FOB (Free On Board) or CIF (Cost, Insurance, and Freight) can affect the total landed cost of products.
Buyer Tips
-
Negotiation: Engage in discussions with multiple suppliers to understand market rates and leverage competitive offers. Building a strong relationship with suppliers can also lead to better pricing and terms.
-
Cost-Efficiency: Evaluate the Total Cost of Ownership (TCO) rather than just the purchase price. Consider factors such as longevity, maintenance, and the frequency of replacements when assessing overall costs.
-
Pricing Nuances for International Buyers: Buyers from regions like Africa, South America, the Middle East, and Europe should be aware of currency fluctuations and local market conditions that can impact pricing. Establishing contracts in stable currencies may mitigate some financial risks.
-
Market Awareness: Stay informed about market trends and technological advancements that could affect pricing and availability. Being proactive can help buyers adapt to changes and secure favorable terms.
Disclaimer
Prices mentioned are indicative and can vary based on market conditions, supplier negotiations, and specific buyer requirements. Always seek multiple quotes and conduct thorough due diligence when sourcing dental implant o rings.
Spotlight on Potential dental implant o rings Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘dental implant o rings’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for dental implant o rings
Understanding the technical properties and terminology associated with dental implant o rings is essential for B2B buyers in the international market. This knowledge not only aids in making informed purchasing decisions but also enhances supplier negotiations and ensures product compatibility.
Critical Specifications for Dental Implant O Rings
-
Material Grade
– Definition: Refers to the quality and type of material used in the manufacturing of the o rings, such as rubber, silicone, or specialty polymers.
– B2B Importance: Material grade impacts durability, flexibility, and biocompatibility. Buyers must ensure that the chosen material meets the specific needs of their clientele, especially in regions with varying patient conditions. -
Tolerance
– Definition: The allowable variation in dimensions of the o ring, typically expressed in millimeters or micrometers.
– B2B Importance: Tolerance affects the fit and retention of the o ring within the dental prosthetic. Inaccurate tolerances can lead to improper seating, which may cause discomfort or failure in the prosthetic. Understanding tolerance is crucial for maintaining high-quality standards. -
Retention Force
– Definition: The amount of force required to remove the o ring from its seat.
– B2B Importance: Retention force is critical for ensuring that the dental prosthetic remains securely in place. Buyers should consider the specific needs of their patient demographics when selecting o rings with varying retention forces. -
Shelf Life
– Definition: The duration for which the o rings maintain their intended performance characteristics when stored properly.
– B2B Importance: A longer shelf life can reduce inventory costs and waste, making it essential for buyers to evaluate the shelf life of products, especially in markets with limited distribution networks. -
Color Coding
– Definition: A system where o rings are differentiated by color to indicate varying retention forces or material properties.
– B2B Importance: Color coding allows for quick identification and selection of the appropriate o ring, streamlining the workflow in busy dental practices. This feature can enhance operational efficiency, particularly in high-volume clinics.
Common Trade Terminology
-
OEM (Original Equipment Manufacturer)
– Definition: A company that produces components that are used in another company’s product.
– Relevance: Understanding OEM relationships is crucial for buyers to establish reliable supply chains and ensure that the components meet their specifications and quality standards. -
MOQ (Minimum Order Quantity)
– Definition: The smallest quantity of a product that a supplier is willing to sell.
– Relevance: Knowing the MOQ helps buyers manage inventory levels and cash flow effectively, particularly in emerging markets where demand may fluctuate. -
RFQ (Request for Quotation)
– Definition: A document sent to suppliers requesting a quote for specific products or services.
– Relevance: Issuing an RFQ allows buyers to compare pricing, terms, and delivery schedules from multiple suppliers, ensuring competitive procurement practices. -
Incoterms (International Commercial Terms)
– Definition: A set of predefined international trade terms that clarify the responsibilities of buyers and sellers in the delivery of goods.
– Relevance: Familiarity with Incoterms is vital for B2B buyers to understand shipping responsibilities, costs, and risks, enabling better negotiation and planning in international transactions. -
Lead Time
– Definition: The time it takes from placing an order to receiving the product.
– Relevance: Understanding lead times is essential for inventory management and ensuring that dental practices are adequately stocked with necessary components. -
Biocompatibility
– Definition: The ability of a material to perform with an appropriate host response when applied within the body.
– Relevance: Biocompatibility is crucial in dental applications, as it affects patient safety and satisfaction. Buyers should prioritize suppliers who provide biocompatible products to enhance the quality of care offered to patients.
By grasping these specifications and terms, B2B buyers can make informed decisions that align with their operational needs and patient expectations, ensuring successful procurement of dental implant o rings.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the dental implant o rings Sector
Market Overview & Key Trends
The dental implant o rings sector is currently experiencing significant growth, driven by several global factors. Increasing demand for dental implants and prosthetics, particularly in emerging markets in Africa, South America, and the Middle East, is reshaping the landscape. The global contract manufacturing market for dental implants and prosthetics is projected to grow from USD 1.3 billion in 2023 to USD 2.27 billion by 2030, reflecting a robust compound annual growth rate (CAGR) of 8.3%. This growth is fueled by the rising prevalence of dental ailments, which necessitates advanced solutions, including high-quality o rings for overdentures.
B2B buyers are increasingly leveraging technology to enhance sourcing strategies. The adoption of digital platforms for procurement processes is transforming traditional supply chains, allowing buyers to access a wider range of suppliers and products. Technologies such as CAD/CAM and 3D printing are also revolutionizing the manufacturing processes, enabling faster production times and customization options that cater to specific clinical needs. Furthermore, the trend towards outsourcing manufacturing to specialized contract manufacturers is becoming prevalent, allowing companies to focus on core competencies while benefiting from cost efficiencies and advanced manufacturing capabilities.
For international buyers, understanding regional pricing dynamics and regulatory requirements is essential. Sourcing o rings that comply with local and international standards not only ensures quality but also fosters trust with end-users. As competition intensifies, buyers must prioritize strategic partnerships with suppliers who can offer both reliability and innovation, thereby enhancing their market position.
Sustainability & Ethical Sourcing in B2B
Sustainability has become a critical consideration in the sourcing of dental implant o rings. As environmental concerns rise globally, buyers are increasingly evaluating the ecological impact of their procurement decisions. The production of dental components, including o rings, can involve processes that generate waste and utilize non-renewable resources. Therefore, adopting sustainable sourcing practices is essential for reducing carbon footprints and promoting environmental stewardship.
Ethical supply chains are gaining traction, with buyers prioritizing suppliers that adhere to sustainable practices. This includes sourcing materials that are environmentally friendly and obtaining certifications that demonstrate a commitment to green practices. Look for suppliers offering biodegradable or recyclable materials for o rings, which can significantly minimize environmental impact.
Moreover, the importance of transparency in the supply chain cannot be overstated. Buyers should seek suppliers who can provide traceability of their materials, ensuring that they are ethically sourced. This not only enhances brand reputation but also meets the growing demand from consumers for responsible business practices. Implementing these sustainability initiatives will not only align with global trends but also position businesses competitively in the market.
Brief Evolution/History
The evolution of dental implant o rings has been driven by advancements in material science and manufacturing technologies. Historically, rubber o rings were the standard, offering basic retention and compatibility. However, as dental practices evolved, so did the need for more durable and versatile solutions. The introduction of silicone and titanium-based o rings marked a significant shift, providing enhanced longevity and performance.
In recent years, innovations such as color-coded retention systems have emerged, allowing for quick customization based on clinical needs. The incorporation of advanced technologies like 3D printing has further accelerated this evolution, enabling more complex designs and faster production times. This historical progression reflects the ongoing commitment to improving patient outcomes through better prosthetic solutions, emphasizing the importance of sourcing high-quality, reliable o rings in the dental industry.
Related Video: International Trade Explained
Frequently Asked Questions (FAQs) for B2B Buyers of dental implant o rings
-
What should I consider when vetting suppliers for dental implant o rings?
When vetting suppliers, prioritize their manufacturing capabilities, quality assurance processes, and industry certifications. Look for suppliers with a proven track record in dental products, preferably with ISO certifications that demonstrate adherence to international quality standards. Request samples to evaluate the product quality firsthand, and consider their responsiveness and customer service as indicators of reliability. Additionally, check for reviews and testimonials from other B2B buyers in your region to gauge their reputation. -
Can I customize dental implant o rings to meet specific requirements?
Yes, many suppliers offer customization options for dental implant o rings, such as varying sizes, colors, and materials to suit different applications. When discussing customization, clearly outline your requirements, including any specific retention forces or compatibility with existing implants. Be aware that customized products may involve longer lead times and higher costs, so factor this into your procurement strategy. Always request prototypes to ensure they meet your specifications before placing larger orders. -
What are typical minimum order quantities (MOQs) and lead times for dental implant o rings?
MOQs and lead times can vary significantly between suppliers and depend on the complexity of the product. For standard nylon-insert o rings, MOQs may range from 100 to 1,000 units, while specialized products could have higher MOQs. Lead times typically range from 4 to 12 weeks, depending on manufacturing capacity and customization requirements. It’s crucial to communicate your needs clearly and negotiate terms that align with your inventory management strategies. -
What payment terms are common when sourcing dental implant o rings internationally?
Payment terms can vary by supplier but typically include options such as advance payment, net 30/60/90 days, or letters of credit for larger orders. It’s advisable to establish clear terms upfront to avoid disputes later. Consider using escrow services or third-party platforms for transactions to mitigate risk, especially when dealing with new suppliers. Ensure that all payment terms are documented in the purchase agreement to protect your interests. -
How can I ensure quality assurance and certification for dental implant o rings?
To ensure product quality, request certifications from suppliers that demonstrate compliance with international standards, such as ISO 13485 for medical devices. Ask for documentation regarding their manufacturing processes, including quality control measures, testing protocols, and traceability of materials used. Conducting an on-site audit or third-party inspection can further verify their quality assurance practices, especially for suppliers located in regions with less stringent regulatory oversight.

Illustrative Image (Source: Google Search)
-
What logistical considerations should I keep in mind when importing dental implant o rings?
Logistical considerations include shipping methods, customs regulations, and potential tariffs. Choose a reliable freight forwarder with experience in medical device shipments to navigate complex customs procedures. Ensure that your supplier provides accurate shipping documentation to avoid delays. Additionally, consider the implications of delivery times on your inventory management, especially if you are sourcing from international markets. -
How can I handle disputes with suppliers effectively?
To handle disputes, establish clear communication channels and document all interactions with suppliers. If issues arise, start with a direct conversation to resolve misunderstandings amicably. If necessary, refer to the terms outlined in your contract regarding dispute resolution procedures, such as mediation or arbitration. Maintaining a professional demeanor and focusing on solutions can often lead to satisfactory outcomes without escalating the situation. -
What are the common challenges faced when sourcing dental implant o rings from international suppliers?
Common challenges include language barriers, cultural differences in business practices, and varying quality standards across regions. Additionally, shipping delays and customs issues can complicate procurement timelines. To mitigate these risks, invest time in building strong relationships with suppliers, utilize technology for real-time communication, and stay informed about international trade regulations affecting your sourcing strategy. Regularly assessing supplier performance can also help identify and address potential issues proactively.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for dental implant o rings
As the global dental implant market continues to grow, the strategic sourcing of dental implant o rings has never been more critical for B2B buyers across Africa, South America, the Middle East, and Europe. Understanding the diverse types of o rings—ranging from standard nylon-insert to titanium-abutment systems—enables buyers to make informed decisions that align with their operational needs and patient expectations.
Key Takeaways for Buyers:
– Quality Matters: Prioritize suppliers that adhere to rigorous quality assurance protocols to ensure product reliability and patient satisfaction.
– Cost vs. Value: While initial costs may vary, consider the long-term benefits of durability and reduced maintenance associated with premium options like extended-life silicone o rings.
– Market Awareness: Stay informed about regional pricing trends and regulatory requirements to optimize procurement strategies.
In an increasingly competitive landscape, the ability to adapt sourcing strategies will be pivotal. By leveraging insights from this guide, buyers can streamline their operations and enhance their service offerings.
Call to Action: Engage with trusted suppliers, explore innovative materials, and continuously assess your sourcing strategies to remain ahead in the evolving dental market. Your commitment to quality and strategic procurement will ultimately define your success in delivering exceptional patient care.
