Introduction: Navigating the Global Market for medical necessity for dental implants
In today’s rapidly evolving global healthcare landscape, the medical necessity for dental implants has emerged as a pivotal concern for dental professionals and their patients alike. For international B2B buyers, particularly those from regions such as Africa, South America, the Middle East, and Europe—including countries like Spain and Egypt—understanding the nuances of dental implant requirements is essential for effective sourcing and procurement. Dental implants are not merely cosmetic enhancements; they serve critical functions in restoring oral health, preventing bone loss, and improving overall quality of life. This guide provides a comprehensive overview of the various types of dental implants, materials used, manufacturing quality control standards, and key suppliers in the market.
Navigating the complexities of medical necessity involves recognizing when implants are required due to health-related issues, such as trauma or disease. This guide will empower B2B buyers with actionable insights into costs associated with dental implants, insurance coverage nuances, and financing options available. Additionally, it addresses frequently asked questions to demystify the decision-making process. By equipping international buyers with this knowledge, we aim to foster informed sourcing decisions that enhance patient outcomes and streamline procurement strategies in the dynamic dental implant market.
Understanding medical necessity for dental implants Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Functional Necessity | Implants required for basic oral function (e.g., chewing) | Dental clinics, hospitals, insurance firms | Pros: Essential for patient health; Cons: Higher costs for complex cases. |
| Aesthetic Necessity | Implants for improving appearance and self-esteem | Cosmetic dentistry, dental tourism | Pros: Enhances patient satisfaction; Cons: Often not covered by insurance. |
| Preventive Necessity | Implants to prevent further oral health deterioration | Dental health organizations, insurers | Pros: Long-term cost savings; Cons: Requires thorough assessment. |
| Trauma-Induced Necessity | Implants needed after accidents or injuries | Emergency dental services, rehabilitation | Pros: Urgent need can expedite procedures; Cons: Complex cases may require multiple consultations. |
| Medical Condition Necessity | Implants due to health issues (e.g., cancer, severe gum disease) | Specialized clinics, hospitals | Pros: May be covered by medical insurance; Cons: Requires comprehensive medical documentation. |
Functional Necessity
Functional necessity for dental implants arises when patients lose teeth essential for basic oral functions like chewing and speaking. This type is particularly relevant for patients who have experienced significant tooth loss due to decay or periodontal disease. B2B buyers should consider the implications of functional necessity on patient health outcomes, as these implants can significantly improve quality of life. Dental clinics and hospitals often need to stock various implant types to address this necessity, ensuring they can cater to a diverse patient base.
Aesthetic Necessity
Aesthetic necessity pertains to implants placed primarily for cosmetic reasons, aimed at improving a patient’s smile and overall appearance. This type is prevalent in cosmetic dentistry and dental tourism, where patients seek treatments that enhance their self-esteem. B2B buyers in this sector should focus on the latest implant technologies and materials that offer both durability and aesthetic appeal. While these implants can lead to high patient satisfaction, they often fall outside insurance coverage, impacting pricing strategies for clinics.
Preventive Necessity
Preventive necessity involves placing implants to avert further oral health issues, such as bone loss or misalignment caused by missing teeth. This type is crucial for maintaining long-term dental health and can lead to significant cost savings by avoiding more complex treatments later. B2B buyers, particularly in dental health organizations and insurance sectors, should evaluate the long-term benefits of preventive implants in their offerings. This requires a thorough assessment of patient needs and may necessitate the integration of preventive care programs.
Trauma-Induced Necessity
Trauma-induced necessity arises when implants are needed following accidents or injuries that result in tooth loss. This type often requires urgent care and is typically addressed by emergency dental services. B2B buyers in this space should prioritize rapid response capabilities and a range of implant options to cater to urgent needs. While the immediate need can facilitate quicker treatment paths, the complexity of trauma cases may require multiple consultations and follow-ups, increasing operational demands.
Medical Condition Necessity
Medical condition necessity refers to implants required due to underlying health issues, such as cancer or severe gum disease. This type often necessitates comprehensive medical documentation and may be eligible for coverage under medical insurance plans. B2B buyers in specialized clinics and hospitals should focus on building relationships with medical professionals to ensure a collaborative approach to patient care. Understanding the medical landscape and the specific needs of patients with chronic conditions can enhance service offerings and improve patient outcomes.
Related Video: DENTAL IMPLANTS : TYPES, SURFACE TREATMENTS, why TITANIUM is used for making implants?
Key Industrial Applications of medical necessity for dental implants
| Industry/Sector | Specific Application of Medical Necessity for Dental Implants | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Healthcare Providers | Replacement of lost teeth due to injury or disease | Enhances patient quality of life and satisfaction | Ensure compliance with local regulations and standards |
| Dental Practices | Support for prosthetic devices like dentures and bridges | Expands service offerings and patient base | Source high-quality, biocompatible materials |
| Insurance Companies | Coverage of medically necessary implants | Reduces long-term healthcare costs for complex cases | Develop clear criteria for medical necessity assessments |
| Rehabilitation Centers | Use in patients recovering from oral surgeries or trauma | Improves recovery outcomes and overall patient health | Collaborate with dental specialists for tailored solutions |
| Medical Device Manufacturers | Production of innovative implant technologies | Drives market competitiveness and technological advancement | Invest in R&D for cutting-edge materials and techniques |
Healthcare Providers
In healthcare settings, dental implants are often deemed medically necessary for patients who have lost teeth due to injury or disease. This application improves not only the functionality of the mouth but also the overall well-being of patients by addressing issues like speech and chewing difficulties. For international buyers, especially in regions like Africa and the Middle East, understanding local healthcare regulations and patient demographics is crucial for sourcing appropriate dental implant solutions.
Dental Practices
Dental practices can leverage the medical necessity of dental implants to support prosthetic devices such as dentures and bridges. By offering these solutions, practices can enhance their service offerings and attract a broader patient base. Buyers in Europe, particularly in Spain, should focus on sourcing high-quality, biocompatible materials that comply with EU regulations to ensure patient safety and satisfaction.
Insurance Companies
Insurance companies play a pivotal role in the coverage of medically necessary dental implants. By establishing clear criteria for what constitutes medical necessity, these companies can help reduce long-term healthcare costs associated with untreated dental issues. B2B buyers in South America should consider partnerships with dental professionals to develop comprehensive guidelines that align with local healthcare practices and policies.
Rehabilitation Centers
Rehabilitation centers can utilize dental implants in patients recovering from oral surgeries or trauma. The integration of implants aids in improving recovery outcomes, providing patients with a functional and aesthetic solution that enhances their quality of life. Buyers from Africa and Europe must collaborate with dental specialists to ensure the implants meet the specific needs of their patient population, including considerations for varying health conditions.
Medical Device Manufacturers
Medical device manufacturers are increasingly focused on producing innovative implant technologies that address the growing demand for dental solutions. By investing in research and development, these manufacturers can stay competitive in the market and meet the evolving needs of healthcare providers. For international B2B buyers, particularly from the Middle East, sourcing cutting-edge materials and techniques is essential for delivering high-quality dental implants that comply with local regulations.
Related Video: How to Perform Dental Implants by MIS -Tutorial (3D Dental Animation)
Strategic Material Selection Guide for medical necessity for dental implants
When selecting materials for dental implants deemed medically necessary, it is crucial to consider their properties, advantages, and limitations. Here, we analyze four common materials used in dental implants: Titanium, Zirconia, PEEK (Polyether Ether Ketone), and Stainless Steel. Each material has unique characteristics that can significantly impact their application in dental implant procedures.
Titanium
Key Properties: Titanium is known for its excellent biocompatibility, corrosion resistance, and strength-to-weight ratio. It can withstand high temperatures and pressures, making it suitable for various implant applications.
Pros & Cons: Titanium implants are highly durable and have a long track record of success in dental applications. However, they can be more expensive than other materials, and their manufacturing process can be complex due to the need for precision machining.
Impact on Application: Titanium’s compatibility with human bone allows for osseointegration, which is critical for the stability of dental implants. This property makes it a preferred choice for patients requiring implants due to tooth loss from disease or injury.
Considerations for International Buyers: Buyers from regions like Africa and South America should be aware of the varying standards for titanium quality. Compliance with ASTM standards is essential to ensure the reliability of the implants.
Zirconia
Key Properties: Zirconia is a ceramic material known for its aesthetic appeal and high strength. It is also highly resistant to wear and corrosion, making it suitable for dental applications.
Pros & Cons: The primary advantage of zirconia is its tooth-like appearance, which is particularly appealing for anterior implants. However, it is generally more brittle than titanium, which can lead to fractures under stress. Additionally, zirconia implants often have a higher initial cost.
Impact on Application: Zirconia is particularly suitable for patients concerned about aesthetics, as it can blend well with natural teeth. However, its brittleness may limit its use in areas of high occlusal force.
Considerations for International Buyers: Buyers in Europe, particularly Spain, should ensure that zirconia implants meet the European Union’s stringent regulations for dental materials.
PEEK (Polyether Ether Ketone)
Key Properties: PEEK is a high-performance polymer known for its excellent mechanical properties and biocompatibility. It is lightweight, resistant to heat, and has good chemical resistance.
Pros & Cons: PEEK offers a flexible alternative to traditional metal implants, which can be beneficial in certain applications. However, it is less commonly used in dental implants, and its long-term performance compared to titanium is still under evaluation.
Impact on Application: PEEK’s flexibility can help reduce stress on surrounding bone structures, potentially leading to better outcomes in specific cases. However, its lower strength compared to metals may limit its use in load-bearing applications.
Considerations for International Buyers: Buyers in the Middle East should be aware of the emerging standards for polymer-based implants, as regulations may vary significantly between countries.
Stainless Steel
Key Properties: Stainless steel is known for its strength, corrosion resistance, and affordability. It is often used in temporary implants or in pediatric dentistry.
Pros & Cons: The cost-effectiveness of stainless steel makes it an attractive option for many dental practices. However, it lacks the biocompatibility and aesthetic qualities of titanium and zirconia, which can be a drawback for permanent implants.
Impact on Application: Stainless steel is primarily used in temporary applications or for patients who may not be candidates for more expensive options. Its use in permanent implants is limited due to concerns about corrosion and aesthetic appearance.
Considerations for International Buyers: Buyers from South America should ensure that stainless steel implants comply with local health regulations, as the quality can vary widely.
Summary Table
| Material | Typical Use Case for medical necessity for dental implants | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Titanium | Permanent dental implants for osseointegration | Excellent biocompatibility | Higher cost, complex manufacturing | High |
| Zirconia | Anterior implants for aesthetic purposes | Tooth-like appearance | Brittle, higher initial cost | Med |
| PEEK | Flexible implants, temporary solutions | Lightweight, chemical resistance | Lower strength compared to metals | Med |
| Stainless Steel | Temporary implants, pediatric applications | Cost-effective | Poor aesthetic, corrosion risk | Low |
This strategic material selection guide provides valuable insights for international B2B buyers, enabling them to make informed decisions regarding dental implants that meet medical necessity criteria. Understanding the properties and implications of each material is crucial for ensuring successful patient outcomes and compliance with regional standards.
In-depth Look: Manufacturing Processes and Quality Assurance for medical necessity for dental implants
Manufacturing dental implants that are deemed medically necessary involves a series of meticulous processes and stringent quality control (QC) measures. For international B2B buyers, particularly in regions such as Africa, South America, the Middle East, and Europe, understanding these processes is essential for ensuring the reliability and safety of the products they procure.
Manufacturing Processes
The production of dental implants typically encompasses four main stages: material preparation, forming, assembly, and finishing. Each stage employs specific techniques to ensure the final product meets the necessary medical standards.
1. Material Preparation
The manufacturing of dental implants often begins with the selection of biocompatible materials, such as titanium or zirconia. These materials are chosen for their strength and ability to integrate with bone.
- Key Techniques:
- Sintering: This process involves heating the material to a temperature below its melting point, allowing particles to bond and form a solid mass.
- Surface Treatment: Techniques like sandblasting or acid-etching are used to enhance the implant’s surface for improved osseointegration.
2. Forming
In this stage, the prepared materials are shaped into the required implant design.
- Key Techniques:
- CNC Machining: Computer Numerical Control (CNC) machining is utilized to create precise shapes and dimensions.
- Additive Manufacturing: 3D printing technologies may be employed to produce complex geometries that enhance the implant’s functionality.
3. Assembly
Once individual components are formed, they are assembled to create the complete implant system.
- Key Techniques:
- Welding and Bonding: Components may be welded or bonded together using medical-grade adhesives to ensure structural integrity.
- Quality Checks: As components are assembled, immediate QC checks are conducted to identify any defects before proceeding.
4. Finishing
The final stage involves polishing and coating the implants to ensure they are smooth and free from contaminants.
- Key Techniques:
- Electropolishing: This method is used to enhance the surface finish, making it smoother and more resistant to corrosion.
- Coating: Biocompatible coatings may be applied to promote better integration with bone tissue.
Quality Assurance
Quality assurance is critical in the manufacturing of dental implants, especially when they are classified as medically necessary. Adherence to international standards and specific industry regulations is paramount.
Relevant International Standards
- ISO 9001: This standard outlines requirements for a quality management system (QMS) and is applicable across various industries, including medical devices.
- ISO 13485: Specifically for medical devices, this standard focuses on ensuring that organizations consistently meet regulatory requirements and customer expectations.
- CE Marking: In Europe, dental implants must comply with the Medical Devices Regulation (MDR) to obtain CE marking, indicating conformity with health, safety, and environmental protection standards.
QC Checkpoints
Quality control involves several checkpoints throughout the manufacturing process:
- Incoming Quality Control (IQC): Raw materials are inspected upon arrival to ensure they meet specified standards.
- In-Process Quality Control (IPQC): Ongoing checks are conducted during manufacturing to detect any deviations from established processes.
- Final Quality Control (FQC): Finished products undergo comprehensive testing to verify that they meet all specifications before shipment.
Common Testing Methods
Several testing methods are employed to ensure the quality and safety of dental implants:
- Mechanical Testing: Evaluates the strength and durability of the implants under simulated physiological conditions.
- Biocompatibility Testing: Assesses how the materials interact with biological tissues.
- Sterility Testing: Ensures that the implants are free from harmful microorganisms.
Verifying Supplier Quality Control
B2B buyers must take proactive steps to verify the quality control measures of their suppliers:
- Audits: Conducting regular audits of suppliers can provide insights into their manufacturing processes and compliance with standards.
- Quality Reports: Requesting detailed quality reports and documentation can help assess the supplier’s adherence to international standards.
- Third-Party Inspections: Engaging independent third-party organizations to conduct inspections can add an additional layer of assurance regarding product quality.
QC and Certification Nuances for International Buyers
For B2B buyers from Africa, South America, the Middle East, and Europe, understanding the nuances of quality control and certification is crucial:
- Regulatory Variations: Different regions may have varying regulations regarding medical devices. Buyers should familiarize themselves with local requirements to ensure compliance.
- Certification Recognition: Not all certifications are universally recognized. Understanding which certifications are accepted in the target market can prevent compliance issues.
- Cultural Considerations: Different regions may have unique expectations regarding product quality and safety. Buyers should consider local standards and practices when assessing suppliers.
In conclusion, a thorough understanding of the manufacturing processes and quality assurance protocols for dental implants deemed medically necessary is essential for international B2B buyers. By focusing on these aspects, buyers can ensure that they procure high-quality products that meet both regulatory standards and patient needs.
Related Video: Dental Implant Procedure | Medical Animation
Comprehensive Cost and Pricing Analysis for medical necessity for dental implants Sourcing
Analyzing the costs and pricing for dental implants classified as medically necessary involves a detailed understanding of various components that contribute to the overall expenditure. For international B2B buyers, particularly those from Africa, South America, the Middle East, and Europe, grasping these nuances is crucial for effective sourcing and budgeting.
Cost Components
- Materials: The type of materials used for dental implants significantly impacts costs. Titanium remains the gold standard due to its biocompatibility, but alternatives such as zirconia are gaining traction. The choice between these materials can vary based on regional availability and market demand.
Illustrative Image (Source: Google Search)
-
Labor: Skilled labor costs are another significant factor. The expertise required for implantology varies greatly across different regions. In countries with high labor costs, such as those in Western Europe, prices will naturally be higher compared to regions with lower labor costs.
-
Manufacturing Overhead: This includes the indirect costs associated with production, such as utilities, rent, and administrative expenses. Efficient manufacturing processes can reduce these overheads, impacting the final price.
-
Tooling: Specialized tools and equipment for producing dental implants can be a substantial investment. The amortization of these costs is factored into the pricing of implants. Suppliers that invest in advanced technology may offer better quality implants but at a higher price point.
-
Quality Control (QC): Rigorous quality assurance processes are critical, especially for medical devices. The costs associated with QC can add to the overall pricing, but they are essential for compliance with international standards and regulations.
-
Logistics: Shipping and handling costs can vary widely based on geographical location and selected Incoterms. Import duties and taxes must also be considered, particularly for buyers in Africa and South America, where tariffs can significantly increase costs.
-
Margin: Finally, suppliers must build a profit margin into their pricing. This margin can vary based on market competition, brand reputation, and the perceived value of the implants.
Price Influencers
Several factors can influence the price of dental implants:
- Volume/MOQ (Minimum Order Quantity): Bulk purchases often lead to discounts, making it beneficial for larger dental practices or organizations.
- Specifications and Customization: Custom implants or those with specific design features typically carry a higher price. Standardized products may be more cost-effective.
- Material Quality and Certifications: Implants that meet higher standards or come with certifications (like ISO or CE marks) may be priced higher due to the assurance of quality.
- Supplier Factors: Established suppliers with a track record of reliability may charge a premium, whereas newer or less reputable manufacturers might offer lower prices.
- Incoterms: Understanding the implications of chosen Incoterms (e.g., FOB, CIF) is essential for calculating total landed costs.
Buyer Tips
For international buyers, especially from regions with varying economic conditions, here are actionable insights:
- Negotiate: Leverage volume purchases to negotiate better terms and prices. Building a long-term relationship with suppliers can also yield benefits.
- Cost-Efficiency: Evaluate the Total Cost of Ownership (TCO) rather than just the upfront costs. Consider long-term durability and potential warranty claims when assessing value.
- Pricing Nuances: Be aware of currency fluctuations and their impact on pricing. Establish clear payment terms to mitigate financial risks.
- Regulatory Compliance: Ensure that any implants sourced comply with local regulations and standards. This can prevent costly delays or rejections upon importation.
Disclaimer
Prices for dental implants can vary significantly based on the factors mentioned above and may change based on market conditions. Buyers are advised to conduct thorough research and consult multiple suppliers to obtain accurate and competitive pricing.
Spotlight on Potential medical necessity for dental implants Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘medical necessity for dental implants’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for medical necessity for dental implants
Key Technical Properties for Medical Necessity in Dental Implants
Understanding the essential technical properties of dental implants is crucial for B2B buyers in the healthcare sector. These specifications help ensure that the implants meet medical necessity criteria and provide long-term solutions for patients.
-
Material Grade
Dental implants are primarily made from titanium or zirconia. Titanium is favored for its biocompatibility and strength, while zirconia offers a more aesthetic solution. The material grade affects the implant’s durability and integration with bone, which is vital for successful outcomes. -
Surface Roughness
The texture of the implant surface plays a significant role in osseointegration—the process by which the implant fuses with the bone. A rougher surface increases the surface area for bone contact, promoting better stability. Specifying the right surface roughness can minimize the risk of implant failure. -
Diameter and Length
Implants come in various diameters and lengths to accommodate different anatomical needs. Selecting the appropriate dimensions is critical for achieving optimal stability and support for prosthetic teeth. Ensuring the availability of various sizes can meet the diverse needs of patients, enhancing the overall treatment efficacy. -
Tolerance Levels
Tolerance refers to the allowable deviation in implant dimensions. High precision is required to ensure a proper fit between the implant and the surrounding bone. Consistent tolerance levels reduce the risk of complications and ensure that the implant performs as intended. -
Load-Bearing Capacity
This property indicates how much force an implant can withstand during function. Understanding the load-bearing capacity is vital for making informed decisions about the type of implant to use, especially in cases where patients may have specific functional demands or habits that could affect implant longevity. -
Corrosion Resistance
Given that dental implants are exposed to the oral environment, corrosion resistance is crucial. Implants must withstand the acidic conditions of the mouth without degrading. Selecting implants with high corrosion resistance ensures longevity and minimizes the risk of complications.
Common Trade Terms in Dental Implant Procurement
Familiarity with industry jargon can streamline communication and enhance negotiation processes for international buyers.
-
OEM (Original Equipment Manufacturer)
Refers to companies that produce components or products that are marketed by another company. In dental implants, understanding OEM relationships can help buyers ensure quality and reliability in the products they procure. -
MOQ (Minimum Order Quantity)
This term indicates the smallest quantity of a product that a supplier is willing to sell. Knowing the MOQ helps buyers plan their purchasing strategy, particularly in managing inventory and costs effectively. -
RFQ (Request for Quotation)
An RFQ is a standard business process where buyers request price quotes from suppliers for specific products. Providing detailed specifications in an RFQ can lead to better pricing and terms, which is essential for budgeting in healthcare procurement. -
Incoterms (International Commercial Terms)
These are pre-defined commercial terms published by the International Chamber of Commerce (ICC) that clarify the responsibilities of buyers and sellers. Familiarity with Incoterms helps buyers understand shipping, risk management, and cost implications in international transactions. -
CE Marking
This indicates that a product meets European Union (EU) safety, health, and environmental protection standards. For buyers in Europe, ensuring that dental implants carry CE marking is essential for compliance and patient safety. -
Warranty Period
The warranty period specifies the duration for which the manufacturer guarantees the product’s performance. Understanding warranty terms is crucial for buyers, as it impacts the long-term viability of their investment in dental implants.
By grasping these technical properties and trade terminologies, international B2B buyers can make informed decisions, ensuring the procurement of dental implants that meet medical necessity standards while optimizing their operational efficiency.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the medical necessity for dental implants Sector
Market Overview & Key Trends
The dental implant market is experiencing robust growth driven by several global factors, including an increasing geriatric population, rising awareness of oral health, and advancements in dental technology. For international B2B buyers, particularly those from Africa, South America, the Middle East, and Europe, understanding these dynamics is crucial. The demand for dental implants is expected to rise, particularly in regions with emerging economies, where dental care is becoming more accessible.
One of the key trends in the sector is the integration of digital technologies into dental implant procedures. Technologies such as 3D imaging, digital impressions, and computer-aided design (CAD) are streamlining the implant process, enhancing precision, and improving patient outcomes. These innovations not only reduce the time and cost involved in procedures but also increase patient satisfaction, thus driving demand further.
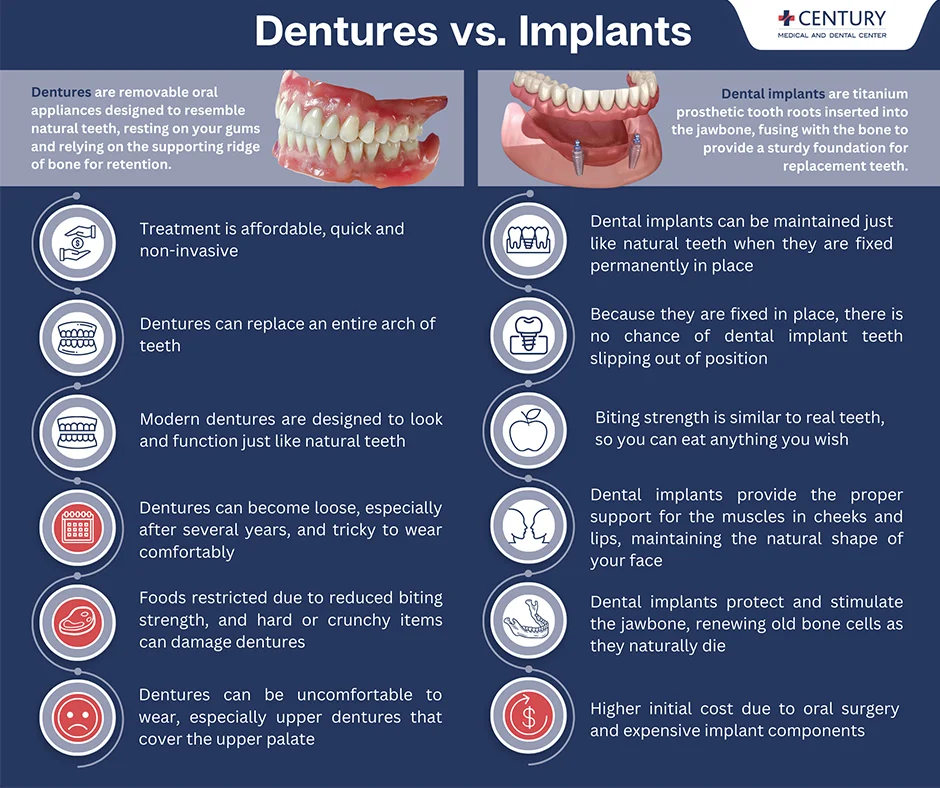
Illustrative Image (Source: Google Search)
Moreover, international buyers should be aware of the growing trend towards value-based care. This shift emphasizes the medical necessity of dental implants, as they are increasingly recognized for their role in preventing further health complications, such as bone loss and nutritional deficiencies. As insurers begin to cover more medically necessary procedures, the market for dental implants is poised for expansion, particularly among older populations and patients with specific health conditions.
Sustainability & Ethical Sourcing in B2B
Sustainability is becoming a paramount consideration in the dental implant sector, as buyers increasingly seek suppliers that prioritize environmental responsibility. The sourcing of dental implants often involves materials like titanium and zirconia, which have significant environmental impacts during extraction and processing. B2B buyers must evaluate their suppliers’ sustainability practices, including waste management, energy use, and the carbon footprint associated with production.
Ethical sourcing is also gaining importance, with consumers and practitioners alike demanding transparency in the supply chain. Ensuring that materials are sourced responsibly not only aligns with corporate social responsibility (CSR) objectives but also enhances brand reputation. Buyers should look for suppliers that hold certifications such as ISO 14001 (Environmental Management) and use eco-friendly materials wherever possible.
Investing in ‘green’ technologies and materials can also provide competitive advantages. For instance, some manufacturers are developing biodegradable options or using recycled materials in their products. This not only reduces environmental impact but can also cater to a growing market of environmentally conscious consumers and healthcare providers.
Brief Evolution/History
The concept of dental implants has evolved significantly over the past several decades. Initially, the focus was primarily on aesthetics and functionality; however, the understanding of dental implants as a medical necessity has gained traction. Historical practices often involved rudimentary techniques and materials, with limited success rates.
In recent years, advancements in materials science and implant technology have transformed the industry. The introduction of biocompatible materials, improved surgical techniques, and post-operative care protocols have greatly enhanced the success rates of implants. As a result, dental implants are increasingly recognized not just as cosmetic enhancements but as essential components of comprehensive healthcare, especially for patients suffering from severe dental loss due to trauma or disease.
This evolution presents significant opportunities for B2B buyers to engage with innovative suppliers who are at the forefront of this transformation, ensuring they provide the most effective and sustainable solutions in the market.
Frequently Asked Questions (FAQs) for B2B Buyers of medical necessity for dental implants
-
What are the key factors to consider when vetting suppliers for dental implants?
When vetting suppliers for dental implants, prioritize their certifications and compliance with international standards such as ISO 13485 for medical devices. Investigate their production capabilities, including the ability to customize implants for specific patient needs. Request samples to assess quality and ensure they have a robust quality assurance process in place. Additionally, evaluate their track record in international trade and their ability to provide references from other B2B clients. -
Can I customize dental implants to meet specific patient requirements?
Yes, many suppliers offer customization options for dental implants, allowing you to tailor the implants to individual patient needs. This could include variations in size, shape, and material. When discussing customization, ensure that the supplier has the necessary technical expertise and equipment to produce the desired specifications. It’s advisable to request detailed documentation and prototypes to validate the customization process. -
What is the typical minimum order quantity (MOQ) for dental implants, and how does it affect pricing?
The MOQ for dental implants varies by supplier but typically ranges from 10 to 100 units per order. Lower MOQs may be available but could lead to higher per-unit costs. Consider negotiating with suppliers for better pricing based on larger orders or long-term contracts. Additionally, assess your inventory management capabilities to determine the most cost-effective approach to sourcing implants. -
What are the lead times for obtaining dental implants from international suppliers?
Lead times for dental implants can vary significantly depending on the supplier’s location, production capacity, and customization requirements. Generally, expect lead times of 4 to 12 weeks. It is crucial to communicate your timeline needs upfront and ask for a production schedule to ensure timely delivery. Consider the potential impact of customs clearance and logistics on lead times when planning your orders. -
How can I ensure the quality assurance and certification of dental implants?
To ensure quality assurance, request documentation that verifies compliance with relevant certifications, such as CE marking in Europe or FDA approval in the U.S. Engage in conversations with suppliers about their quality control processes, including how they handle testing and validation of their products. It may also be beneficial to conduct on-site audits or request third-party inspection reports to gain further confidence in their quality practices. -
What logistics considerations should I keep in mind when importing dental implants?
Logistics considerations include understanding customs regulations, import duties, and shipping methods. Work with suppliers who have experience in international shipping and can provide guidance on documentation required for customs clearance. Additionally, consider the reliability of the chosen shipping method to minimize delays. Evaluate the supplier’s ability to handle logistics and any associated costs to ensure a smooth import process. -
How should disputes regarding quality or delivery be handled with suppliers?
Establish clear terms and conditions in your purchase agreements to outline the procedures for handling disputes. In case of a quality issue, document the problem with evidence, such as photographs or inspection reports, and communicate it promptly to the supplier. Aim for resolution through open dialogue, and consider mediation or arbitration as a last resort if issues cannot be resolved amicably. Building a strong relationship with your suppliers can also facilitate smoother dispute resolution. -
What payment options are available for sourcing dental implants internationally?
Payment options may vary by supplier, but common methods include wire transfers, letters of credit, and PayPal. Some suppliers may offer financing options or payment plans for larger orders. It’s essential to discuss payment terms upfront, including deposits and final payment timelines, to avoid misunderstandings. Consider using escrow services for high-value transactions to enhance security and mitigate risks associated with international payments.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for medical necessity for dental implants
In conclusion, understanding the medical necessity for dental implants is vital for international B2B buyers in the dental industry. The strategic sourcing of dental implants should focus on proven medical justifications, such as the prevention of bone loss, improvement in oral function, and enhancement of overall health. Buyers must prioritize suppliers that can provide clear documentation and support for the medical necessity of their products, ensuring compliance with varying insurance requirements across regions.
Key takeaways include the importance of drafting comprehensive medical necessity letters and understanding the specific eligibility criteria for coverage. By establishing strong partnerships with reputable manufacturers and suppliers, buyers can enhance their product offerings while addressing the critical needs of patients.
Looking ahead, the demand for dental implants is expected to grow, driven by increasing awareness of oral health and advancements in implant technology. Now is the time for B2B buyers from Africa, South America, the Middle East, and Europe to leverage strategic sourcing practices that align with these trends. Engage with innovative suppliers today to ensure your offerings meet the evolving needs of the market, securing a competitive advantage in this essential healthcare sector.
