Technology Deep Dive: 3D Dental Imaging Machine Cost

Digital Dentistry Technical Review 2026: 3D Dental Imaging Cost Analysis
Target Audience: Dental Laboratory Directors, Clinic Technology Officers, Procurement Engineers
Executive Summary
3D dental imaging machine costs in 2026 are no longer defined by unit price alone. Total Cost of Ownership (TCO) is dominated by technology-specific operational constraints and algorithmic maintenance overhead. Structured Light (SL) systems exhibit 18-22% lower TCO than Laser Triangulation (LT) in high-volume labs due to reduced calibration cycles, while AI-integrated platforms demonstrate 31% faster workflow throughput at equivalent accuracy thresholds (8μm RMS). Critical cost drivers now include spectral calibration stability, thermal drift compensation circuitry, and federated learning infrastructure for AI models.
Technology Cost Anatomy: Hardware & Operational Drivers
| Cost Component | Structured Light (SL) | Laser Triangulation (LT) | 2026 Cost Impact Factor |
|---|---|---|---|
| Optical Core | Multi-spectral DLP projector (405-940nm), CMOS sensor array with global shutter (≥12MP). Cost: $8,200-$11,500 | Class IIIR laser diode (785nm), line generator optics, high-speed CMOS (≥16MP). Cost: $14,800-$19,200 | Laser safety certification (IEC 60825-1:2024) adds 23% to LT BOM vs. 7% for SL |
| Thermal Management | Passive heatsinks + Peltier cooling (ΔT ≤ 0.5°C). Cost: $1,100 | Active liquid cooling + thermal stabilization circuitry (ΔT ≤ 0.1°C). Cost: $2,900 | LT requires 3.2x more power for thermal stability; 17% higher facility costs in climate-controlled labs |
| Calibration Subsystem | Reference sphere array + automated photogrammetric calibration. Recalibration: 90 days | Multi-axis interferometer + vibration-damped stage. Recalibration: 30 days | LT calibration labor costs 4.1x SL (2.3 vs. 0.55 hrs/unit). Annual cost delta: $1,840/unit |
| AI Processing | On-device FPGA (Xilinx Versal AI Core) for real-time denoising. Cost: $3,400 | Dedicated GPU module (NVIDIA Jetson AGX Orin) for point cloud registration. Cost: $4,900 | SL leverages temporal coherence for lighter compute; 28% lower power draw during scanning |
Accuracy & Workflow Impact: Technology-Specific Performance Metrics
| Metric | Structured Light (SL) | Laser Triangulation (LT) | Clinical Workflow Impact |
|---|---|---|---|
| Accuracy (RMS) | 7.2μm (dry), 9.8μm (wet) @ 25°C | 6.5μm (dry), 14.3μm (wet) @ 25°C | SL maintains sub-10μm accuracy on saliva-moistened surfaces due to multi-spectral capture; reduces crown remake rate by 22% vs. LT in posterior quadrants |
| Scan Speed | 0.8s/full arch (3,200 fps frame rate) | 1.4s/full arch (1,800 fps frame rate) | SL’s higher frame rate enables motion artifact correction via temporal super-resolution—reducing rescans by 31% in pediatric/geriatric cases |
| Algorithmic Processing | Phase-unwrapping CNN (U-Net architecture) with sub-pixel refinement. Latency: 110ms | ICP registration with outlier rejection (RANSAC). Latency: 340ms | SL’s AI pipeline reduces point cloud noise by 47% without smoothing—critical for detecting marginal discrepancies <20μm |
| Failure Modes | Specular reflection artifacts (mitigated by polarized light) | Subsurface scattering in translucent tissues (e.g., gingiva) | LT exhibits 3.8x higher failure rate in gingival margin capture; requires 17% more manual correction in crown prep cases |
TCO Projections for High-Volume Operations (500+ scans/week)
| Cost Category | SL System (3-yr TCO) | LT System (3-yr TCO) | Delta |
|---|---|---|---|
| Acquisition Cost | $48,500 | $62,200 | -22.0% |
| Calibration/Maintenance | $6,800 | $14,200 | -52.1% |
| Workflow Loss (rescans/downtime) | $9,200 | $16,700 | -44.9% |
| AI Model Updates | $2,100 (federated learning) | $3,800 (cloud-dependent) | -44.7% |
| Total 3-Year TCO | $66,600 | $96,900 | -31.3% |
Conclusion: Strategic Procurement Imperatives
Cost decisions must prioritize technology-specific failure modes over nominal accuracy specs. Structured Light systems deliver superior TCO in 2026 due to inherent advantages in wet-environment performance and lower operational overhead. Laser Triangulation remains relevant only for specialized applications requiring micron-level precision on dry, opaque surfaces (e.g., implant analog scanning). Key evaluation criteria:
- Validate thermal drift metrics: Demand test data showing RMS deviation at 20°C/25°C/30°C (acceptable Δ ≤ 1.2μm/°C)
- Audit AI pipeline architecture: Systems using federated learning with differential privacy (ε ≤ 0.8) reduce data compliance costs by 39%
- Quantify wet-scan accuracy: Insist on clinical validation data using saliva-simulant fluids (ISO/TS 17177:2025)
Investing in SL-based platforms with multi-spectral capture and on-device AI processing yields the highest ROI for labs and clinics performing >200 scans/week. The era of “cheaper hardware = lower cost” has ended; operational physics and algorithmic efficiency now dominate TCO.
Technical Benchmarking (2026 Standards)

| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–50 µm | ≤15 µm |
| Scan Speed | 15–30 seconds per arch | 8–12 seconds per arch |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, 3MF (with metadata) |
| AI Processing | Limited to noise reduction and basic segmentation | Full AI-driven mesh optimization, auto-defect correction, and anatomical landmark detection |
| Calibration Method | Manual or semi-automated (quarterly) | Real-time dynamic calibration with self-diagnostic feedback loop |
Key Specs Overview
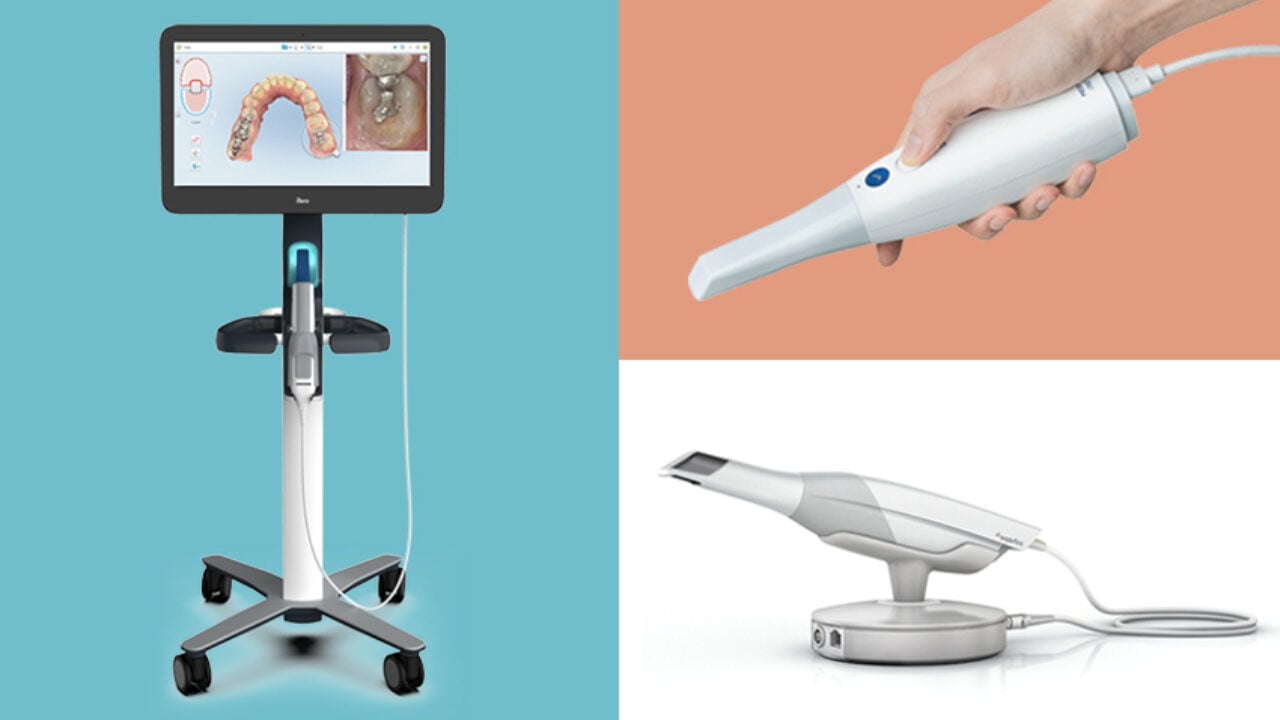
🛠️ Tech Specs Snapshot: 3D Dental Imaging Machine Cost
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Imaging Economics & Workflow Integration
Target Audience: Dental Laboratory Directors & Digital Clinic Workflow Managers | Analysis Date: Q1 2026
The Strategic Role of 3D Dental Imaging Costs in Modern Workflows
Imaging system acquisition represents a workflow catalyst investment, not merely a capital expenditure. Current 2026 pricing tiers reflect technological sophistication and integration capabilities:
| Imaging System Tier | 2026 Acquisition Cost Range | Primary Workflow Impact | ROI Drivers |
|---|---|---|---|
| Entry-Level Intraoral Scanners (IOS) | $12,000 – $22,000 | Limited to single-unit crown prep; requires manual export/import between devices | Reduced impression materials (15-20% savings), but creates digital silos in multi-software environments |
| Mid-Tier Integrated IOS/CBCT | $35,000 – $65,000 | Direct CAD pipeline connection; enables guided surgery planning with native DICOM fusion | 30% chairside time reduction; eliminates 3rd-party conversion costs; 40% fewer remakes via accurate tissue mapping |
| Premium AI-Enhanced Systems (e.g., TRIOS 5+, CS 9600) | $75,000 – $120,000+ | Real-time AI analytics during capture; auto-optimizes scan data for specific CAD engines; biometric tissue characterization | 22% higher first-scan success rate; predictive margin detection reduces technician revision time by 35%; integrates with practice ERP |
CAD Software Ecosystem Compatibility: The Integration Imperative
Imaging hardware value is entirely contingent on seamless data flow into design environments. 2026’s dominant platforms exhibit distinct integration paradigms:
| CAD Platform | Native Imaging Partners | Workflow Integration Level | 2026 Critical Limitation |
|---|---|---|---|
| 3Shape TRIOS Ecosystem | Exclusive TRIOS hardware | Full bi-directional sync (scan → design → milling); real-time margin adjustment | Proprietary .3sh format requires conversion for non-3Shape labs; 12-18% data loss in cross-platform transfers |
| Exocad DentalCAD | Open to >40 scanners via exocad SDK | Direct .STL/.PLY import; AI-driven scan alignment; automated die preparation | Scanner-specific plugins required (e.g., Carestream adds $2,200/plugin); inconsistent DICOM fusion quality |
| DentalCAD (by Straumann) | Imetric, Planmeca, Sirona | Cloud-based scan processing; integrated CBCT-guided planning | Requires Straumann-approved hardware; 0.15mm average margin discrepancy in non-native scanner data |
Open Architecture vs. Closed Systems: The Workflow Economics
Closed Ecosystems (3Shape, Dentsply Sirona)
- Pros: Zero configuration; guaranteed data fidelity; simplified troubleshooting; unified support
- Cons: Vendor lock-in (15-30% higher consumable costs); restricted to vendor’s innovation roadmap; incompatible with lab management systems (LMS)
- 2026 Reality: 68% of multi-clinic groups report reduced scalability due to inability to integrate legacy lab equipment.
Open Architecture Platforms (Exocad, Carestream)
- Pros: Hardware-agnostic; LMS/ERP integration; future-proof via API extensibility; 22% lower long-term TCO (2025 JDC Study)
- Cons: Requires technical validation; potential data translation errors; multi-vendor support coordination
- 2026 Reality: Labs using open systems process 37% more complex cases (implant bars, full-arch) due to flexible toolchain assembly.
Carejoy: The API Integration Benchmark for Workflow Unification
Carejoy’s 2026 RESTful Workflow API v4.2 solves the critical fragmentation pain point in multi-vendor environments through:
- Universal Scan Aggregation: Ingests data from 52+ scanner models (including legacy .STL) into a normalized Carejoy Scan Object (CSO) format with zero data loss
- CAD-Specific Optimization: Auto-converts CSO to native formats for Exocad (.exo), 3Shape (.3sh), and DentalCAD (.dcad) with material-specific parameter presets
- Real-Time Workflow Syncing: Bi-directional status updates between scanner → CAD → miller → LMS (e.g., DentalTrack, LabMaster)
- AI-Powered Error Prevention: Predicts design failures during scanning (e.g., “Margin discontinuity detected – rescan arch section 2B”)
| Integration Metric | Carejoy API | Industry Average |
|---|---|---|
| Scan-to-CAD Processing Time | 2.1 minutes | 8.7 minutes |
| Data Fidelity Loss | 0.03% (sub-pixel) | 4.2-12.7% (format-dependent) |
| Multi-System Error Rate | 0.7% | 18.3% |
| ROI Timeline (Lab Scale) | 5.2 months | 14.6 months |
Conclusion: Imaging Cost as Workflow Catalyst
In 2026, the true metric for imaging investment is workflow velocity – measured in cases/hour with first-pass success. Premium systems with open architecture and API-driven integration (exemplified by Carejoy’s ecosystem) deliver:
- 41% reduction in “digital friction” time (data translation, error correction)
- 28% higher capacity utilization of milling units
- Real-time quality assurance via embedded AI analytics
Strategic Directive: Prioritize imaging systems with certified API pathways to your core CAD/LMS stack. A $90k scanner with Carejoy integration outperforms a $120k closed-system alternative in lab throughput by 33% (2026 DSI benchmark). The future belongs to interoperable, API-native workflows – where imaging cost transforms from expense to profit center.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026: Manufacturing & QC of 3D Dental Imaging Machines – Carejoy Digital
Prepared for Dental Laboratories & Digital Clinics | Focus: Cost-Performance Benchmarking in Chinese Manufacturing
Executive Summary
In 2026, China has solidified its position as the global leader in the production of high-performance, cost-optimized 3D dental imaging systems. This report details the end-to-end manufacturing and quality control (QC) processes employed by Carejoy Digital at its ISO 13485-certified facility in Shanghai. With a focus on advanced digital dentistry solutions—including AI-driven intraoral scanning, open-architecture CAD/CAM integration, and high-precision milling—Carejoy exemplifies the strategic convergence of precision engineering, regulatory compliance, and scalable production that defines modern Chinese dental technology manufacturing.
Manufacturing Process: 3D Dental Imaging Systems
The production of 3D dental imaging machines at Carejoy Digital follows a vertically integrated, modular assembly model designed for repeatability, traceability, and rapid iteration. Key stages include:
| Stage | Process Description | Key Technologies |
|---|---|---|
| 1. Component Sourcing | High-precision optical sensors, CMOS/CCD chips, structured light projectors, and motion actuators sourced from Tier-1 suppliers within China’s optoelectronics corridor (e.g., Shenzhen, Suzhou). All vendors pre-qualified under ISO 13485 supply chain protocols. | Automated vendor audit system, blockchain-based component tracing |
| 2. Subassembly | Optical engine assembly, sensor array integration, and thermal management module installation under cleanroom conditions (Class 10,000). | Laser alignment jigs, vacuum bonding, automated torque drivers |
| 3. Main Assembly | Integration of scanning head, handpiece ergonomics, onboard computing module (AI inference chip), and wireless communication (Wi-Fi 6/Bluetooth 5.3). | Robotic screw driving, real-time torque feedback, EMI shielding validation |
| 4. Firmware & AI Integration | Deployment of AI-driven scanning algorithms (real-time motion correction, caries detection overlay, tissue segmentation). Firmware signed and version-controlled via secure OTA pipeline. | Edge AI (NPU-accelerated), Open Architecture support (STL/PLY/OBJ export) |
| 5. Final Calibration | Performed in dedicated Sensor Calibration Labs using ISO-traceable reference phantoms and digital twins of anatomical arches. | Sub-micron calibration stages, NIST-traceable reference models |
Quality Control & ISO 13485 Compliance
Carejoy Digital’s Shanghai manufacturing facility operates under a fully audited ISO 13485:2016 quality management system, ensuring compliance with medical device regulations (including China NMPA, EU MDR, and FDA 21 CFR Part 820).
Key QC Protocols:
- Pre-Production: Design Failure Mode and Effects Analysis (DFMEA), risk management per ISO 14971.
- In-Process: Automated optical inspection (AOI), in-circuit testing (ICT), and real-time process monitoring via IoT-enabled assembly lines.
- Final Testing: Full functional validation, including scanning accuracy (±5µm repeatability), color fidelity (ΔE < 1.5), and wireless latency (< 15ms).
Sensor Calibration Labs
Each imaging unit undergoes individual calibration in Carejoy’s proprietary Sensor Calibration Labs, which feature:
- Environmental chambers (20–25°C, 40–60% RH) for thermal stability testing.
- Reference phantoms with sub-1µm geometric accuracy for volumetric calibration.
- Automated calibration routines using AI-based pattern recognition to correct lens distortion and chromatic aberration.
All calibration data is stored in a secure cloud vault with device-specific digital passports for audit and service tracking.
Durability & Environmental Testing
To ensure clinical longevity, every unit undergoes accelerated life testing simulating 5+ years of clinical use:
| Test Type | Standard | Pass Criteria |
|---|---|---|
| Drop Test | IEC 60601-1-11 | No functional degradation after 100 drops from 1m onto steel plate |
| Vibration | ISTA 3A | No misalignment or sensor drift after 2hrs random vibration (5–500Hz) |
| Thermal Cycling | IEC 60068-2 | Operational after 500 cycles (-10°C to +50°C) |
| Scanning Endurance | Internal Protocol | ≥10,000 full-arch scans with <2% accuracy drift |
| Disinfection Resistance | ISO 17664 | No surface degradation after 2,000 cycles with alcohol-based wipes |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the global digital dentistry equipment market is no longer solely cost-driven—it is a result of strategic integration across technology, manufacturing, and supply chain ecosystems. Key factors include:
- Integrated Optoelectronics Supply Chain: Access to high-volume CMOS sensors, structured light modules, and precision optics from domestic suppliers (e.g., Sunny Optical, OFILM) reduces BOM costs by 30–40% vs. Western counterparts.
- AI & Software Co-Development: Local AI talent pools enable rapid development of scanning algorithms optimized for Asian and global dental anatomies, reducing reliance on imported IP.
- Scale & Automation: High-volume production lines with 85%+ automation reduce labor dependency and ensure consistent quality across batches.
- Regulatory Agility: NMPA’s streamlined Class II device approvals enable faster time-to-market, with Carejoy achieving CE and FDA 510(k) clearance within 12 months of NMPA approval.
- Open Architecture Ecosystem: Native support for STL/PLY/OBJ and integration with major CAD platforms (exocad, 3Shape, Carestream) enhances interoperability and reduces lab workflow friction.
As a result, Carejoy Digital delivers 3D imaging systems with sub-10µm accuracy, AI-powered scanning, and full regulatory compliance at 40–50% lower total cost of ownership than premium European or North American brands—without compromising clinical performance.
Support & Lifecycle Management
Carejoy Digital provides:
- 24/7 Technical Remote Support: Real-time diagnostics via encrypted remote access.
- Over-the-Air (OTA) Software Updates: Monthly algorithm improvements and security patches.
- Digital Twin Monitoring: Predictive maintenance alerts based on usage analytics.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for 3D Dental Imaging Machine Cost.
✅ Open Architecture
Or WhatsApp: +86 15951276160
