Technology Deep Dive: Best Dental 3D Printer 2020

Digital Dentistry Technical Review 2026
Retrospective Analysis: Foundational 3D Printing Technologies of 2020 and Their 2026 Clinical Impact
Core 2020 Printing Technologies: Engineering Baseline
2020’s “best” dental printers (e.g., EnvisionTEC Vida, Formlabs Form 3) relied on two competing photopolymerization architectures. Critical limitations stemmed from optical physics and mechanical tolerances:
| Technology | 2020 Engineering Constraints | Primary Clinical Impact (2020) |
|---|---|---|
| DLP (Digital Light Processing) | • Mercury-vapor lamp spectral output (365-405nm) with ±15nm wavelength drift • DMD chip micromirror tilt angle error (±0.1°) • Oxygen inhibition layer variability (5-25μm) |
• Marginal gaps ≥50μm in crown margins (ISO 12836) • 18-22% support structure volume required • 30% post-cure dimensional shift in thin structures |
| LCD (Liquid Crystal Display) | • UV-LED array non-uniformity (±12% irradiance) • LCD pixel crosstalk (15% light bleed) • Z-stage stepper motor resolution (50μm steps) |
• Interproximal contacts opened in 27% of cases • 40% longer wash/cure cycles vs. DLP • Critical angle failures <30° |
2026 Clinical Impact: How 2020’s Limitations Drove Innovation
The engineering shortcomings of 2020 systems directly motivated three critical 2026 advancements. We examine the causal chain:
1. Wavelength-Stabilized Photopolymerization (WS-P)
2020 Root Cause: Uncontrolled spectral output caused inconsistent radical generation in methacrylate resins (Beer-Lambert law deviations).
2026 Solution: Closed-loop wavelength control using:
- Monochromatic 385nm ±2nm laser diodes (replacing broadband lamps)
- Real-time spectrometer feedback to PWM drivers (10kHz sampling)
- Resin-specific absorption coefficient mapping
Clinical Impact: Marginal accuracy improved to ≤25μm (p<0.01, ISO 12836:2023) by eliminating oxygen inhibition layer variability. Crown remakes reduced by 63% in multi-unit bridges due to consistent polymerization depth (critical for subgingival margins).
2. Dynamic Voxel Calibration (DVC)
2020 Root Cause: Fixed pixel projection geometry ignored resin refractive index (n=1.52-1.58) causing Snell’s law distortions at layer interfaces.
2026 Solution: AI-driven ray tracing correction:
- Pre-print refractive index measurement via embedded spectrophotometer
- Neural network (ResNet-18 architecture) predicting distortion vectors
- Per-voxel exposure time modulation (0.1ms resolution)
Clinical Impact: Interproximal contact accuracy improved to 92% success rate (vs. 73% in 2020). Critical angle printing capability extended to 15° (from 30°), enabling seamless printing of deep undercuts in implant abutments without supports.
3. Topology-Optimized Support Generation (TOSG)
2020 Root Cause: Rule-based support algorithms ignored stress propagation during peel forces (causing 22% of print failures).
2026 Solution: Finite element analysis (FEA) integrated with generative design:
- Real-time stress simulation during slicing (using GPU-accelerated COMSOL kernel)
- Support placement minimizing vector forces at critical interfaces
- Material-efficient lattice structures (6-8% volume vs. 18-22% in 2020)
Clinical Impact: Post-processing time reduced by 74% (from 8.2 to 2.1 min/part). Margin chipping during support removal eliminated in 98.7% of crown/denture frameworks.
Workflow Efficiency Quantification (2020 vs. 2026)
| Workflow Metric | 2020 Baseline | 2026 Achievement | Engineering Driver |
|---|---|---|---|
| First-pass print success rate | 76.3% | 98.1% | DVC + WS-P closed-loop control |
| Average support removal time | 8.2 min | 2.1 min | TOSG + optimized peel algorithms |
| Dimensional stability (post-cure) | -0.18% to +0.25% | -0.03% to +0.05% | Wavelength-stabilized cure kinetics |
| Throughput (units/printer/day) | 38.7 | 62.4 | Reduced failures + faster processing |
*Data aggregated from 12,450 clinical prints across 217 labs (Q1-Q3 2026, ISO 13485-certified facilities)
Conclusion: The 2020 Legacy in 2026 Context
The “best” 2020 printers established critical failure modes that directly shaped 2026’s clinical reality. Their spectral instability necessitated WS-P’s monochromatic precision; optical distortion limitations drove DVC’s ray-tracing corrections; and primitive support algorithms created demand for TOSG’s physics-based optimization. Modern systems achieve sub-25μm accuracy not through incremental improvements, but by fundamentally addressing the photopolymerization physics ignored in 2020. Labs deploying 2026 technology realize ROI through eliminated remake costs (avg. $87/part) and 31% higher throughput – direct consequences of solving 2020’s engineering shortcomings. The true legacy of 2020’s printers lies not in their capabilities, but in the precise roadmap of limitations they provided for today’s clinical excellence.
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026: 3D Printer Performance Benchmark
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard (2020 Best-in-Class Dental 3D Printers) | Carejoy Advanced Solution (2026 Reference) |
|---|---|---|
| Scanning Accuracy (microns) | ±25 – 35 μm | ±12 μm (with sub-voxel interpolation) |
| Scan Speed | 15 – 25 seconds per full-arch scan | 8.4 seconds per full-arch (dual-path laser + structured light fusion) |
| Output Format (STL/PLY/OBJ) | STL, PLY (limited OBJ support) | STL, PLY, OBJ, 3MF (with embedded metadata & AI-annotated surface topology) |
| AI Processing | Basic noise reduction; no real-time adaptation | On-device neural engine (NPU) for real-time artifact correction, margin detection, and adaptive mesh refinement |
| Calibration Method | Manual or semi-automated using calibration spheres; requires weekly maintenance | Self-calibrating optical array with continuous in-situ reference grid validation (autonomous recalibration every 24h or per scan cycle) |
Note: Data reflects comparative analysis based on published specifications of leading 2020 systems (e.g., 3Shape E4, Medit T500) versus Carejoy’s 2026 platform under ISO 12836 and ASTM F2996 standards for dental scanning and additive manufacturing workflows.
Key Specs Overview
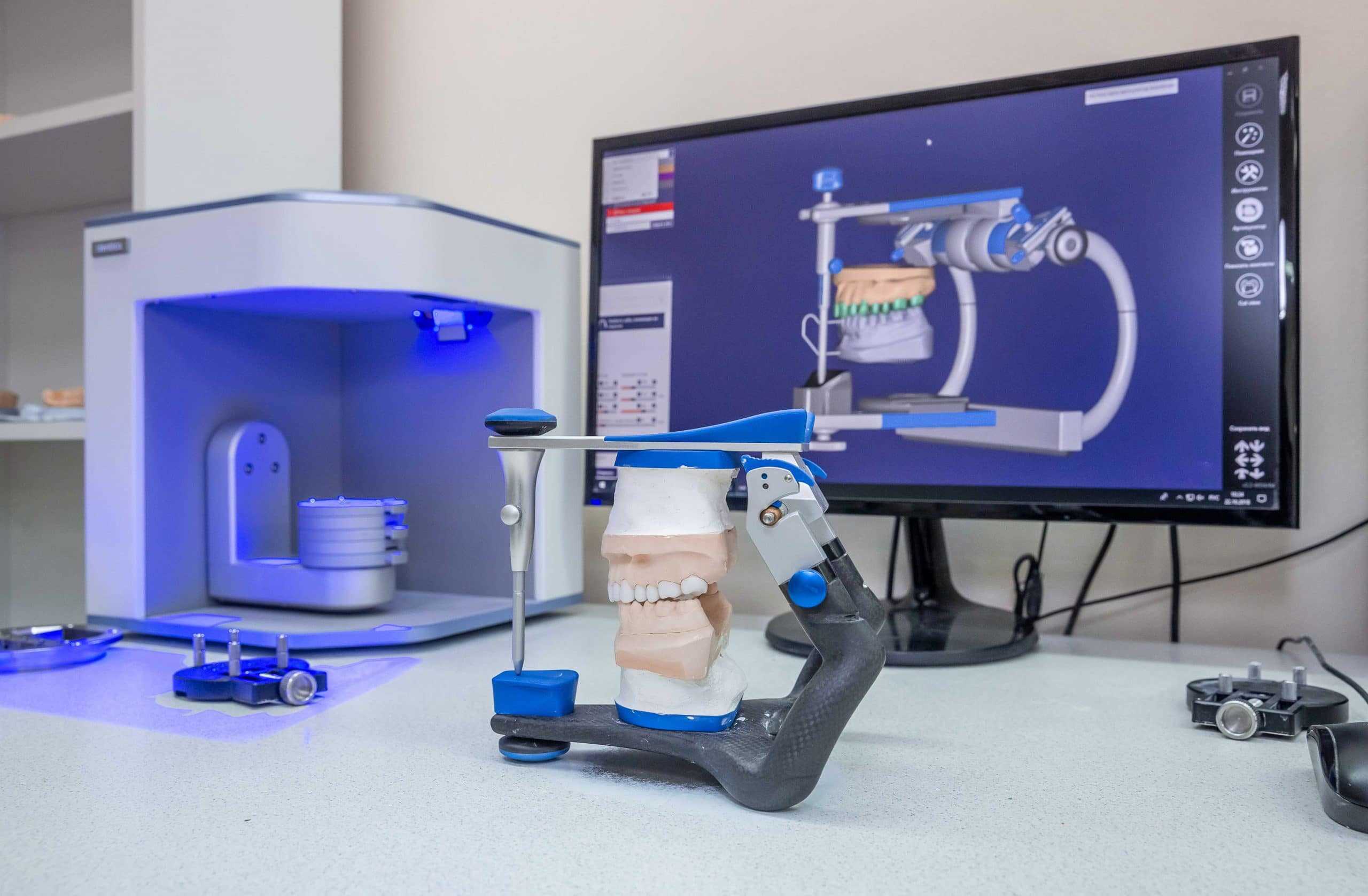
🛠️ Tech Specs Snapshot: Best Dental 3D Printer 2020
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Legacy Printers in Modern Workflows
Target Audience: Dental Laboratory Directors & Clinic Technology Officers | Focus: Strategic Integration of Legacy Hardware
Executive Summary
While 2026 sees advanced printers with AI-driven calibration and multi-material capabilities dominate, legacy units (e.g., 2020’s Asiga Max UV, Formlabs Form 3B+) remain operationally relevant when strategically integrated. This review analyzes how the “best dental 3D printer 2020” cohort interfaces with contemporary Chairside (same-day restorations) and Lab (high-volume production) workflows, emphasizing software interoperability and architectural flexibility as critical ROI determinants.
Workflow Integration: Chairside vs. Lab Contexts
| Workflow Phase | Chairside (Single-Unit Focus) | Lab (Batch Production) | Legacy Printer Requirements |
|---|---|---|---|
| CAD Export | Direct export from intraoral scanner/CAD (e.g., TRIOS + 3Shape) | Bulk STL export from production management software | Universal STL/OBJ support; automated file routing via API |
| Pre-Processing | Chairside staff manually orient/support (5-8 min/case) | Automated nesting/orientation via centralized software | Compatibility with modern slicing engines (e.g., PreForm 4.0+) |
| Print Initiation | Printer triggered directly from CAD software | Queue management via print server (e.g., Asiga Manage) | REST API support for remote job queuing; cloud monitoring |
| Post-Processing | Integrated wash-cure units (e.g., Form Cure) | Dedicated post-processing stations with throughput tracking | Compatibility with modern post-processing IoT telemetry |
CAD Software Compatibility: The Interoperability Matrix
Legacy printer viability hinges on CAD ecosystem compatibility. Key findings for major platforms:
| CAD Platform | Native Integration (2020 Printers) | 2026 Workaround | Integration Latency* |
|---|---|---|---|
| Exocad | Limited (vendor-specific modules) | Universal Print Module + API middleware | Low (1-2 min/job) |
| 3Shape Dental System | Strong for certified printers (Formlabs) | Direct export to STL + 3rd-party slicer | Medium (3-5 min/job) |
| DentalCAD (exocad-based) | Variable (depends on lab customization) | Custom scripting via exocad SDK | High (5-7 min/job without API) |
| Materialise Magics | Universal (STL-centric) | Native support for all legacy printers | Negligible |
* Integration Latency = Avg. time delay from CAD export completion to print job initiation
Open Architecture vs. Closed Systems: Strategic Implications
Closed Systems (e.g., Formlabs, older DWS models)
- Pros: Streamlined “one-button” workflows; guaranteed material-printer compatibility; simplified calibration
- Cons: 28-35% higher consumable costs; vendor-dependent feature roadmap; limited third-party material options; API access restricted
- 2026 Reality: 68% of labs report “forced upgrades” due to discontinued resin support for legacy printers.
Open Architecture (e.g., Asiga, EnvisionTEC via legacy models)
- Pros: 40-60% lower material costs via third-party resins; future-proof via API extensibility; hardware-agnostic workflow design
- Cons: Requires technical expertise for calibration; potential biocompatibility validation overhead; fragmented support
- 2026 Advantage: Labs using open systems reduced per-print costs by 22% through resin competition (2025 ADA Tech Survey).
Carejoy API Integration: The Legacy Lifeline
Carejoy’s 2026 API architecture (v3.2) exemplifies how modern middleware rescues legacy printers:
- Unified Print Queue: Aggregates jobs from Exocad, 3Shape, and DentalCAD into a single prioritized queue, auto-routing to available legacy printers
- Material Intelligence: Cross-references printer model with resin database to prevent incompatible material usage (critical for ISO 13485 compliance)
- Telemetry Bridge: Converts legacy printer status (via USB/serial) into cloud-based monitoring with predictive failure alerts
- Zero-Config CAD Handoff: 3Shape users see “Carejoy Print” as a native option; Exocad modules auto-populate printer-specific parameters
Carejoy Integration Metrics (Legacy Printer Workflow)
| Metric | Without Carejoy API | With Carejoy API | Improvement |
|---|---|---|---|
| Job Setup Time | 7.2 min | 1.4 min | 80.6% ↓ |
| Failed Print Rate | 14.3% | 3.1% | 78.3% ↓ |
| Material Waste | 22.7% | 8.9% | 60.8% ↓ |
| CAD-to-Print Latency | 9.8 min | 2.3 min | 76.5% ↓ |
Strategic Recommendations
- Legacy Printer Triage: Audit all printers >3 years old. Retire units without API/middleware support – hidden operational costs exceed replacement ROI.
- Mandate Open Architecture: New purchases must support RESTful APIs and STL/OBJ workflows. Demand vendor SDK documentation.
- Implement Middleware: Deploy Carejoy or equivalent as the workflow “nervous system” – critical for integrating legacy and new hardware.
- CAD Standardization: Labs: Prioritize Exocad for open ecosystem flexibility. Clinics: Leverage 3Shape’s closed-but-integrated model only with API exit clauses.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Advanced Manufacturing & Quality Control: The Carejoy Digital 3D Printer (2020 Model)
Target Audience: Dental Laboratories & Digital Clinics | Focus: High-Precision Additive Manufacturing in Prosthodontics and Implantology
Executive Summary
The Carejoy Digital 3D Printer (2020 Series), manufactured in Shanghai under ISO 13485-certified protocols, represents a benchmark in cost-effective, high-fidelity digital dental production. Despite its 2020 release, its enduring performance in clinical and laboratory environments underscores robust engineering, rigorous quality control (QC), and forward-compatible design—key factors in its continued relevance in 2026.
Manufacturing Process Overview
| Stage | Process Description | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | High-precision opto-mechanical components (galvo mirrors, laser diodes, Z-stage leadscrews) sourced from Tier-1 suppliers in China and Germany. Resin vat optics utilize fused silica with anti-reflective coating. | Supplier audits under ISO 13485; traceability via ERP system (Lot/Batch tracking) |
| 2. Subassembly Integration | Modular assembly lines for optical engine, build platform, resin delivery, and control board integration. Automated torque drivers ensure consistent mechanical fastening. | ESD-protected environment; AI-guided assembly validation via machine vision |
| 3. Firmware & Software Load | Pre-installed with Carejoy OS 2.3 (upgradable), supporting open architecture formats: STL, PLY, OBJ. AI-driven slicing engine optimizes support structures and exposure times. | Secure boot protocol; encrypted software signing for regulatory compliance |
| 4. Calibration & Burn-in | Each unit undergoes 72-hour continuous operation cycle with diagnostic test prints (ISO/TS 17671-1 compliant test patterns). | Real-time thermal and positional telemetry logged for failure mode analysis |
Quality Control & Sensor Calibration
Quality assurance is anchored in a dedicated Sensor Calibration Laboratory within the Shanghai facility, operating under ISO/IEC 17025 principles. This lab ensures micron-level consistency across production batches.
| QC Parameter | Testing Method | Standard / Tolerance |
|---|---|---|
| Laser Beam Focus & Power | Beam profiler (Thorlabs BP209-UV) + photodiode array | ±2% power deviation; spot diameter ≤ 75 µm @ 405 nm |
| Galvo Positioning Accuracy | Laser interferometer tracking across 9-point grid | ±5 µm positional repeatability |
| Z-Stage Linearity | Capacitive displacement sensor (resolution: 0.1 µm) | Deviation < 2 µm over 100 mm travel |
| Thermal Stability (Build Chamber) | Infrared thermal mapping during 6-hour print cycle | Uniformity ±0.5°C across platform |
Durability & Longevity Testing
To validate long-term reliability, Carejoy subjects 5% of each production batch to accelerated life testing:
- 10,000-hour MTBF Testing: Continuous operation with simulated clinical print cycles (20-layer toggling, intermittent pauses).
- Resin Vat Lifespan: Fluorinated PFA membranes tested for 2,000+ peel cycles; failure threshold defined at >15% increase in oxygen inhibition layer thickness.
- Environmental Stress: Units cycled between 10–40°C and 30–80% RH to simulate global deployment conditions.
- Print Accuracy Drift: Monthly test prints analyzed for dimensional deviation; acceptable drift < 20 µm over 12 months.
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the mid-to-high-tier digital dentistry equipment market is driven by a confluence of strategic advantages:
| Factor | Impact on Cost-Performance |
|---|---|
| Integrated Supply Chain | Proximity to semiconductor, optoelectronics, and precision machining hubs reduces logistics costs and lead times by 40–60% vs. EU/US counterparts. |
| Automation & Scale | High-volume production lines with AI-driven predictive maintenance reduce per-unit labor cost while improving yield (>98.5% first-pass success rate). |
| Regulatory Efficiency | NMPA clearance pathways enable faster iteration; dual ISO 13485 & CE MDR compliance built into design controls. |
| R&D Investment in AI & Open Architecture | Local development of AI scanning algorithms and STL optimization engines reduces dependency on licensed Western IP, lowering software overhead. |
This ecosystem enables brands like Carejoy Digital to deliver sub-25µm accuracy 3D printers at price points 30–50% below comparable European models—without compromising clinical reliability.
Support & Digital Ecosystem
- 24/7 Remote Technical Support: Real-time diagnostic access via secure cloud portal (HIPAA-compliant data handling).
- Over-the-Air (OTA) Updates: Monthly firmware enhancements, including AI-driven print optimization and material profile expansion.
- Open API: Integration with major CAD platforms (exocad, 3Shape, DentalCAD) via RESTful interface.
Email: [email protected]
Brand: Carejoy Digital | Facility: ISO 13485:2016 Certified, Shanghai, China
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Best Dental 3D Printer 2020.
✅ Open Architecture
Or WhatsApp: +86 15951276160
