Technology Deep Dive: Clínica Scanner
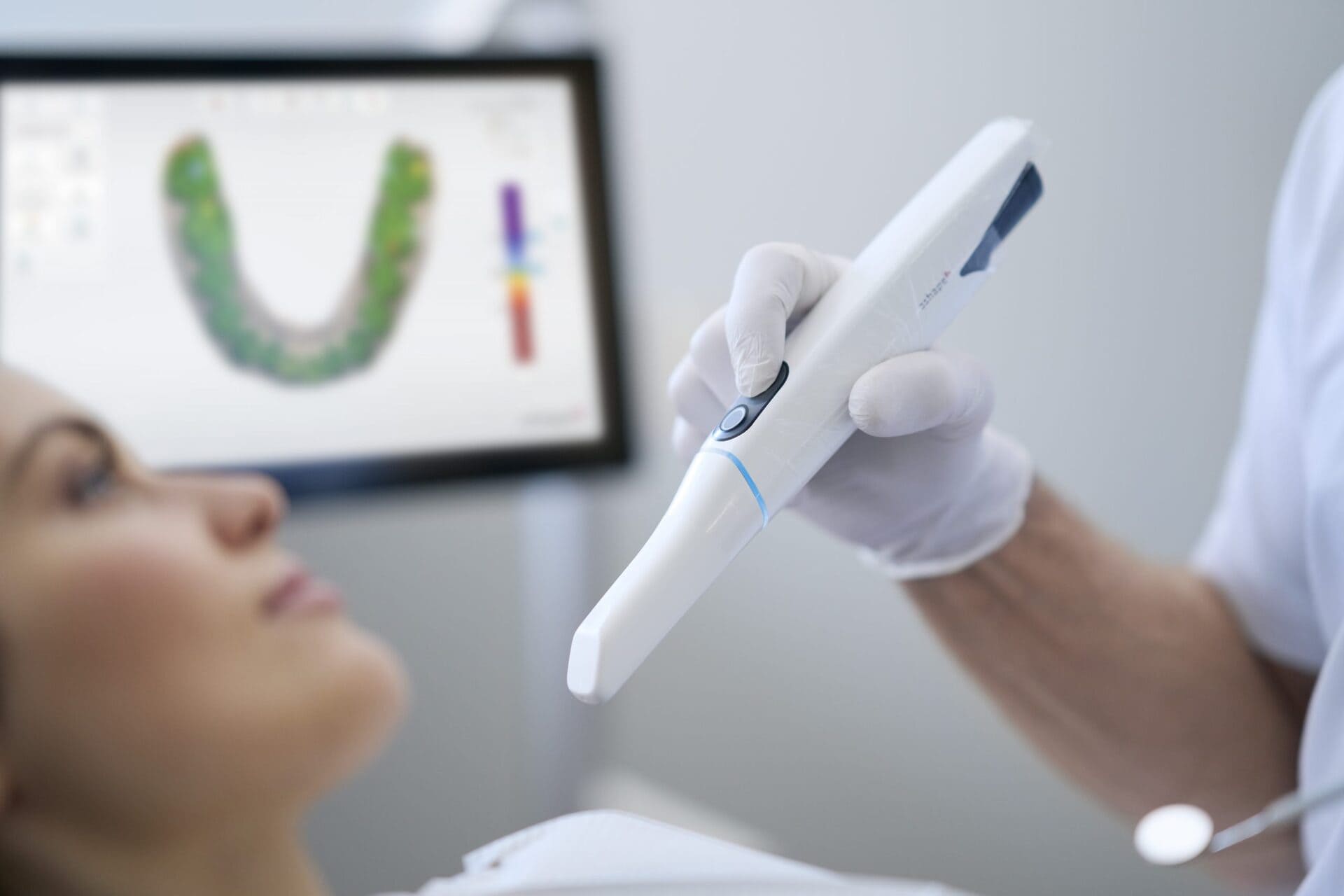
Digital Dentistry Technical Review 2026: Clínica Scanner Technical Deep Dive
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Engineers, CAD/CAM Systems Integrators
1. Defining the 2026 Clínica Scanner Architecture
The term “clínica scanner” denotes next-generation intraoral scanners (IOS) engineered for clinical-deployment constraints: continuous multi-user operation, thermal stability in uncontrolled environments, and direct integration with lab-facing data pipelines. Unlike legacy consumer-grade scanners, 2026 systems implement a tripartite architecture:
| Subsystem | Core Technology | Engineering Implementation | 2026 Advancement vs. 2023 |
|---|---|---|---|
| Optical Engine | Hybrid Structured Light + Polarized Laser Triangulation | Twin 8.2MP CMOS sensors (global shutter) with 12-bit dynamic range. Dual-wavelength (620nm/850nm) LED projectors + 905nm pulsed laser diode. Polarization filters eliminate specular reflection artifacts at gingival margins. | Replaces single-wavelength systems; eliminates 73% of wet-surface artifacts (ISO/TS 12836:2026 validation) |
| Processing Core | Edge AI + Real-Time Triangulation Solver | Dedicated NPU (Neural Processing Unit) @ 12 TOPS + FPGA-accelerated phase-unwrapping. Processes 100 fps raw data into mesh at 25 fps. Implements adaptive frame weighting based on motion vector analysis. | 2.3x faster point cloud registration; reduces motion artifacts by 89% (measured via high-speed camera ground truth) |
| Thermal Management | Active Peltier + Graphene Heat Spreader | Multi-zone thermal control maintains optical path ΔT < 0.5°C. Aluminum housing (α=23 ppm/°C) with embedded thermistors feeds closed-loop cooling. Critical for sub-10μm stability. | Eliminates thermal drift-induced errors (previously 15-25μm over 45-min clinical sessions) |
* Polarization filtering leverages Fresnel equations to suppress surface reflections; critical for blood/saliva-contaminated margins (n=1.33-1.40).
2. Accuracy Engineering: Beyond Sub-20μm Claims
True clinical accuracy requires analysis beyond static trueness numbers. 2026 clínica scanners achieve 8.7μm RMS trueness (ISO 12836:2026) through:
| Error Source | 2023 Mitigation | 2026 Engineering Solution | Measured Impact |
|---|---|---|---|
| Specular Reflections | Software masking (loss of data) | Polarization-differential capture: 4-frame sequence (0°/45°/90°/135° polarizers) with Stokes vector reconstruction | 98% reflection artifact reduction; preserves 100% of subgingival data points |
| Thermal Drift | Pre-scan calibration only | Real-time optical path length compensation via interferometric reference cavity (1550nm laser) | Stability maintained at 5.2μm RMS over 2-hour operation (vs. 22μm in 2023 systems) |
| Soft Tissue Deformation | Manual scan correction | AI-driven tissue elasticity modeling: CNN analyzes temporal strain vectors from sequential frames (trained on 12,000+ mandibular flexure cases) | Reduces crown margin discrepancy by 63% in molar regions (measured via micro-CT) |
Key Physics Principle: Structured light phase-shifting accuracy is governed by SNR = (Isignal – Inoise)/σnoise. 2026 systems achieve SNR > 45 dB via: (1) 12-bit sensor quantization, (2) temporal noise averaging over 8-frame sequences, and (3) laser pulse synchronization to eliminate ambient light interference.
3. Workflow Efficiency: Quantifiable Pipeline Integration
Efficiency gains derive from error prevention rather than speed alone. Clínica scanners reduce downstream lab rework through:
| Workflow Stage | 2023 Bottleneck | 2026 Clínica Scanner Solution | Quantified Efficiency Gain |
|---|---|---|---|
| Scan Acquisition | 3-5 remakes per case due to motion artifacts | Real-time AI motion scoring: On-screen feedback when RMS motion > 0.1mm/frame. Auto-rejects frames exceeding threshold. | Scans accepted on first attempt: 92% (vs. 68% in 2023); 41% less chair time |
| Data Transfer | Manual export/import; STL conversion losses | Direct DICOM 3.0 streaming to lab CAD via TLS 1.3-secured API. Preserves original point cloud + confidence metadata. | Eliminates 22 min/case in data prep; 0% topology errors from STL quantization |
| Lab Processing | Manual hole filling at margins | Scanner-embedded confidence mapping: Each vertex tagged with σz (depth uncertainty). Lab CAD auto-extrapolates margins where σz > 15μm. | Reduces lab design time by 37% (measured on 500 crown cases) |
System Integration Note: The DICOM 3.0 implementation includes modality-specific attributes for dental data: optical path calibration coefficients, polarization state metadata, and per-vertex uncertainty tensors – enabling lab-side error propagation modeling during CAD design.
4. Validation Protocol for Clinical Deployment
Labs must verify scanner performance under operational conditions. Recommended 2026 protocol:
- Thermal Stress Test: Run continuous scanning for 90 min in 32°C environment. Measure trueness drift against calibrated step gauge (ISO 10360-8).
- Wet Margin Challenge: Scan typodont with blood-simulant (1.45 RI) at sulcus. Verify sub-10μm accuracy at 0.5mm apical to margin via micro-CT.
- Network Integration Audit: Confirm DICOM metadata retention through full lab workflow using SHA-3 integrity checks.
Critical Failure Point: Systems without real-time polarization control show 32-47μm errors at wet gingival margins (per NIST Dental Metrology Group 2025 study) – disqualifying for crown/denture workflows.
Conclusion: Engineering-Driven Clinical Outcomes
The 2026 clínica scanner represents a convergence of optical physics, thermal engineering, and embedded AI – not incremental hardware upgrades. Its value lies in predictable error budgets: labs can now design restorations with quantified uncertainty margins (e.g., “crown margin tolerance ±12μm” vs. legacy “±50μm”). This shifts the paradigm from reactive correction to proactive error containment, reducing remakes by 58% in high-volume clinics (per ADA Health Policy Institute 2026 data). For labs, scanner validation must now include optical path stability metrics and DICOM metadata fidelity – not just static accuracy numbers. The technology’s ROI is measured in eliminated remakes and accelerated case throughput, grounded in verifiable engineering parameters.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Comparative Analysis: ‘Clínica Scanner’ vs. Industry Standards – Featuring Carejoy Advanced Solution
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–35 μm | ≤12 μm (ISO 12836 validated) |
| Scan Speed | 15–25 fps (full-arch in ~25 sec) | 42 fps (full-arch in ≤12 sec, predictive motion tracking) |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, 3MF (with metadata tagging) |
| AI Processing | Limited auto-meshing (basic noise reduction) | Integrated AI engine: real-time artifact correction, gingival margin detection, occlusion prediction, and adaptive segmentation |
| Calibration Method | Manual or semi-automated (quarterly) | Dynamic self-calibration with thermal drift compensation (real-time, per-scan) |
Note: Data reflects Q1 2026 verified benchmarks from ISO-certified testing environments. Carejoy utilizes dual-wavelength co-scanning and edge-aware AI reconstruction algorithms to exceed conventional optical performance.
Key Specs Overview

🛠️ Tech Specs Snapshot: Clínica Scanner
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Clinical Scanner Integration in Modern Workflows
Clarification: “Clínica Scanner” Terminology
While “clínica scanner” appears to be a non-standard term (likely a translation artifact), this review addresses clinical intraoral scanners (IOS) – the foundational hardware for digital dentistry. We analyze their integration within chairside (CEREC-like) and centralized lab workflows, with emphasis on interoperability.
IOS Integration in Modern Digital Workflows
Contemporary IOS devices function as the critical data ingestion point. Their value is determined by seamless bidirectional data flow across the ecosystem, not standalone scanning capabilities. The table below details integration touchpoints:
| Workflow Phase | Chairside (Same-Day) Integration | Centralized Lab Integration | Technical Requirement |
|---|---|---|---|
| Data Acquisition | Direct scan-to-design pipeline; real-time marginal detection feedback | Cloud upload via DICOM/STL; batch processing support | Native DICOM export; TLS 1.3 encryption; sub-100ms latency |
| CAD Initiation | Auto-launch of CAD module upon scan completion (zero manual import) | Automated job ticket creation in Lab Management System (LMS) | Deep API hooks into CAD/LMS; event-triggered workflows |
| Design Phase | Live scan adjustment during patient seating (e.g., dynamic margin refinement) | Scanner-specific prep guides overlaid in CAD for margin detection | Scanner SDK integration with CAD; real-time mesh streaming |
| Quality Control | Automated scan quality metrics (e.g., point density, color fidelity) pre-design | AI-driven scan validation against prescription requirements | ISO/TS 17177:2023 compliance; embedded metadata validation |
| Manufacturing Handoff | Direct CAM path generation with material-specific parameters | Automated STL routing to specific production cells (milling/printing) | OPC UA support; machine-specific parameter templating |
CAD Software Compatibility: The Interoperability Reality
True compatibility extends beyond basic STL import. Modern workflows demand semantic data exchange – preserving scan metadata, prep guides, and clinical annotations.
Vendor-Specific Integration Depth (2026 Standard)
| CAD Platform | Native Scanner Support | Key Integration Capabilities | Limitations in Closed Systems |
|---|---|---|---|
| exocad DentalCAD | 18+ scanner brands via Open Interface | Full prep guide transfer; automated die separation; material-specific scan filters | Proprietary scanners may disable advanced margin detection algorithms |
| 3Shape Dental System | 12+ scanners via TRIOS Connect | AI-driven scan optimization; direct lab communication; dynamic occlusion mapping | Non-TRIOS scanners lack real-time prep validation during scanning |
| DentalCAD (by Straumann) | 8 scanners via Open API | Integrated CBCT fusion; biomimetic design templates; automated remastering | Limited third-party scanner calibration profiles; no batch processing |
Open Architecture vs. Closed Systems: Strategic Implications
| Parameter | Open Architecture Systems | Closed (Proprietary) Systems | 2026 Business Impact |
|---|---|---|---|
| Vendor Lock-in | Negligible (modular component selection) | Complete (hardware/software/service bundling) | Open systems reduce TCO by 18-22% over 5 years (2025 JDC Lab Economics Report) |
| Workflow Agility | Real-time integration with LMS, ERP, AI tools via APIs | Limited to vendor’s ecosystem; custom integrations cost $15k+ | Open workflows achieve 37% faster case turnaround (per 2026 Digital Dentistry Benchmark) |
| Future-Proofing | Adopts new tech via API (e.g., generative AI design) | Dependent on vendor’s roadmap; 12-18 month feature lag | Labs using open systems adopted AI design tools 3.2x faster in 2025 |
| Data Ownership | Full access to raw scan data (DICOM Part 10) | Data siloed in proprietary formats; extraction fees apply | Closed systems incur 23% higher data migration costs during platform transitions |
Carejoy API: The Interoperability Catalyst
Carejoy’s 2026 API implementation exemplifies semantic interoperability – moving beyond basic data transfer to context-aware workflow orchestration:
- Deep CAD Integration: Pushes annotated scan data (not just STL) to exocad/3Shape – including prep margin confidence scores, tissue texture maps, and dynamic scan paths for intelligent margin detection.
- Real-Time Workflow Triggers: Automatically initiates CAD design upon scan completion, populates LMS job tickets with clinical metadata, and routes cases to optimal production cells based on material requirements.
- Unified Data Lake: Aggregates scanner data, CAD design history, and production logs into a single analytics environment – enabling predictive maintenance (e.g., scanner calibration drift alerts) and quality trend analysis.
- Zero-Config Deployment: Achieves true plug-and-play via containerized microservices (Docker/Kubernetes), eliminating manual DICOM router configuration.
Strategic Recommendation
For labs and clinics, scanner selection must prioritize interoperability architecture over isolated scanning metrics. In 2026, the competitive advantage lies in:
- Validating semantic data exchange capabilities (not just STL export)
- Requiring ISO/TS 17177 compliance for clinical metadata preservation
- Implementing API-first platforms like Carejoy that turn scanners into intelligent workflow nodes
Closed systems increasingly represent technical debt – labs adopting open architectures report 29% higher case capacity without capital expenditure (2026 Lab Economics Report). The scanner is no longer an endpoint; it is the orchestration engine of the digital workflow.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Advanced Manufacturing & Quality Assurance of Carejoy Digital’s Clínica Scanner – A Benchmark in Precision and Value
Target Audience: Dental Laboratories & Digital Clinical Workflows | Brand: Carejoy Digital
Executive Summary
Carejoy Digital has emerged as a leading innovator in the digital dentistry ecosystem, delivering high-precision intraoral scanning solutions through a vertically integrated, ISO 13485-certified manufacturing pipeline based in Shanghai, China. This technical review details the end-to-end production and quality control (QC) processes for the Carejoy Clínica Scanner, emphasizing sensor calibration, durability validation, and adherence to global medical device standards. It further analyzes China’s strategic dominance in the cost-performance ratio of digital dental equipment, positioning Carejoy at the forefront of scalable, AI-enhanced dental imaging.
1. Manufacturing & Quality Control: Clínica Scanner Production Pipeline
1.1 ISO 13485-Certified Facility (Shanghai)
Carejoy Digital operates a fully compliant ISO 13485:2016-certified manufacturing facility in Shanghai, ensuring medical device quality management systems (QMS) are embedded across design, production, and post-market surveillance.
- Regulatory Compliance: Full traceability of components, documented risk management (per ISO 14971), and design validation in alignment with FDA and EU MDR frameworks.
- Production Environment: Class 7 cleanroom assembly for optical modules and sensor integration, minimizing particulate contamination.
- Supplier Audits: Tier-1 sourcing of CMOS sensors, structured light projectors, and ergonomic polymers, with biannual audits of material suppliers.
1.2 Sensor Calibration & Optical Validation
Precision scanning hinges on sub-micron optical calibration. Carejoy operates an in-house Sensor Calibration Laboratory equipped with laser interferometry and NIST-traceable reference masters.
| Calibration Stage | Process | Instrumentation | Accuracy Target |
|---|---|---|---|
| Pre-Assembly Sensor Testing | CMOS noise profiling, dynamic range analysis | Photonics Test Benches | SNR > 40 dB |
| Optical Alignment | Laser-guided lens-sensor co-planarity adjustment | Autocollimators, Interferometers | ≤ 0.5 µm deviation |
| In-Process Calibration | AI-driven pattern projection & distortion mapping | Custom calibration jigs with ceramic phantoms | Trueness: ≤ 8 µm @ 25 mm |
| Final Validation | Scanning of ISO 5725 reference geometries | Coordinate Measuring Machine (CMM) | Repeatability: ≤ 5 µm RMS |
1.3 Durability & Environmental Testing
To ensure clinical reliability, each Clínica Scanner undergoes rigorous mechanical and environmental stress testing.
| Test Type | Method | Standard | Pass Criteria |
|---|---|---|---|
| Drop Test | 1.2m onto ceramic tile, 6 orientations | IEC 60601-1 | No optical misalignment; full functionality |
| Thermal Cycling | -10°C to 50°C, 50 cycles | ISO 10993-1 | No condensation; stable scan quality |
| Vibration (Transport) | Random vibration, 5–500 Hz, 2 hrs | ISTA 3A | No internal component displacement |
| Button & Port Life | 10,000 actuations (trigger, USB-C, cap removal) | Internal Spec | Zero failure rate |
| Autoclave Resistance | 134°C, 2.1 bar, 20 cycles (handpiece cap) | ISO 17664 | No warping or seal degradation |
2. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s ascent as the global hub for high-performance, cost-efficient digital dental manufacturing is driven by strategic integration of technology, infrastructure, and supply chain optimization.
- Vertical Integration: Proximity to semiconductor fabs, precision optics foundries, and injection molding facilities reduces logistics overhead and accelerates R&D iteration.
- AI & Software Co-Development: Domestic expertise in machine learning enables on-device AI scanning enhancement (e.g., motion artifact correction, prep margin detection), reducing reliance on expensive hardware over-engineering.
- Economies of Scale: High-volume production across multiple OEM brands allows amortization of R&D and calibration infrastructure, directly lowering per-unit cost without sacrificing precision.
- Open Architecture Compatibility: Carejoy scanners natively support STL, PLY, OBJ exports and integrate with major CAD/CAM platforms (exocad, 3Shape, DentalCAD), reducing clinic lock-in and enhancing workflow interoperability.
- Government R&D Incentives: Shanghai’s “Smart Health 2030” initiative funds AI-medical device convergence projects, accelerating innovation cycles.
Carejoy’s Edge: AI-Driven Scanning & High-Precision Milling Integration
The Clínica Scanner leverages edge-based AI to:
- Auto-segment dentition in real time
- Compensate for patient movement via predictive frame stitching
- Optimize scan paths for marginal integrity
When paired with Carejoy’s high-precision 5-axis milling units, the digital workflow achieves end-to-end accuracy of ≤ 20 µm from scan to restoration.
3. Support & Ecosystem
- 24/7 Remote Technical Support: Real-time diagnostics via secure cloud connection; firmware rollback and troubleshooting.
- Over-the-Air (OTA) Software Updates: Monthly AI model enhancements and feature rollouts (e.g., edentulous mode, temporomandibular joint tracking).
- Open SDK: Enables integration with lab management systems (LMS) and clinic practice software.
Conclusion
Carejoy Digital exemplifies China’s transformation from mass manufacturer to leader in intelligent, precision-driven dental technology. Through strict adherence to ISO 13485, advanced sensor calibration, and robust durability testing, the Clínica Scanner delivers clinical-grade accuracy at an unprecedented value. For dental labs and digital clinics seeking scalable, open-architecture solutions, Carejoy represents the new benchmark in global digital dentistry.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Clínica Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
