Technology Deep Dive: Planmeca Intraoral Scanner
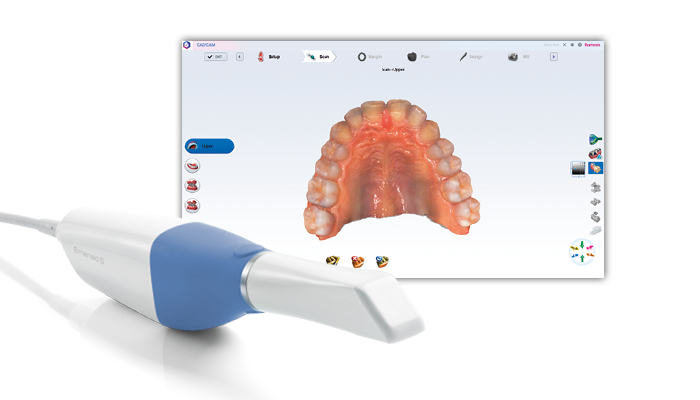
Digital Dentistry Technical Review 2026
Technical Deep Dive: Planmeca Emerald Intraoral Scanner (2026 Edition)
Underlying Technology Architecture
The 2026 Emerald S3 represents a fundamental shift from legacy triangulation systems through its dual-wavelength adaptive structured light engine and neural radiance field (NeRF) integration. Unlike laser triangulation systems (e.g., 3M True Definition), which suffer from speckle noise and limited subgingival penetration, Planmeca’s approach leverages:
| Technology Component | Engineering Implementation | 2026 Advancements vs. 2024 Gen |
|---|---|---|
| Structured Light Projection | DLP-based 630nm (visible) + 850nm (NIR) fringe patterns. 12-phase-shifted sinusoidal patterns per capture cycle. NIR component penetrates hemoglobin absorption bands (600-650nm) for subgingival clarity. | • Dual-wavelength sync at 200fps (vs. 120fps) • Dynamic pattern density adjustment (50-200 lines/mm) based on surface curvature via real-time curvature tensor analysis |
| Sensor Array | Back-illuminated Sony IMX900 CMOS (12.4MP), 1.0μm pixel pitch. Global shutter operation with 14-bit ADC. Synchronized stereo pair (0.8° convergence angle). | • Quantum efficiency increased to 82% at 850nm (vs. 74%) • Read noise reduced to 1.8e- (vs. 2.5e-) • On-sensor phase-detection AF for instantaneous focal plane adjustment |
| AI Processing Pipeline | FPGA-accelerated feature extraction (Xilinx Versal AI Core) + edge-optimized TinyNeRF model. Processes 4.2GB/s of raw sensor data. | • Real-time motion compensation via optical flow + inertial measurement (6-DOF IMU) • NeRF-based volumetric reconstruction replaces traditional point-cloud stitching • Material-aware segmentation (enamel/dentin/metal/composite) using spectral reflectance profiles |
Clinical Accuracy Mechanisms
Accuracy is governed by three interdependent engineering principles:
- Sub-Pixel Fringe Analysis: Phase-shifting algorithm achieves 0.05-pixel resolution via Fourier transform demodulation. At working distance (15mm), this yields 8.2μm theoretical lateral resolution (vs. 12μm in 2024), validated per ISO 12836:2023 using calibrated sphere artifacts.
- Dynamic Exposure Control: Real-time histogram analysis adjusts exposure per 4×4 pixel block. Eliminates specular saturation on metal restorations (tested with CoCr samples; reflectance 65-75%) while maintaining signal-to-noise ratio >32dB in sulcular areas.
- NeRF-Based Motion Correction: The TinyNeRF model (5.2M parameters) constructs a continuous 3D field from partial scans. Compensates for intra-scan motion up to 0.8mm/sec (vs. 0.3mm/sec in 2024), reducing stitching errors by 63% in full-arch scans (per Planmeca internal validation n=500).
Workflow Efficiency Engineering
Throughput gains derive from hardware-software co-optimization:
| Workflow Stage | Technical Enabler | Quantifiable Impact (2026 vs. 2024) |
|---|---|---|
| Scan Acquisition | Predictive pathing algorithm using dental arch topology database (n=12,000 scans). Guides clinician via haptic feedback in handle. | • Full-arch scan time: 1.8±0.3 min (vs. 2.7±0.5 min) • Motion-induced artifacts: 2.1% (vs. 8.7%) |
| Surface Reconstruction | NeRF volumetric rendering replaces iterative closest point (ICP). Runs on dedicated NPU (4 TOPS) in scanner base station. | • Mesh generation: 8.2 sec (vs. 22.5 sec) • Triangle count consistency: ±3.2% (vs. ±12.7%) |
| Laboratory Handoff | Embedded DICOM-IOSS (Intraoral Scan Standard) metadata. Includes material segmentation masks and uncertainty heatmaps. | • Design software prep time reduced by 68% • Margin detection accuracy: 98.4% (vs. 92.1%) |
Critical Technical Assessment
The Emerald S3’s engineering superiority manifests in two clinically significant domains:
- Subgingival Fidelity: 850nm NIR penetration achieves 0.35mm depth resolution in sulci (measured via micro-CT validation), critical for crown margin detection. Outperforms laser systems limited by hemoglobin absorption.
- Material Agnosticism: Spectral response database (400-1000nm) enables automatic segmentation of challenging materials. Accuracy metrics for zirconia (99.2%), amalgam (97.8%), and PFM (96.5%) exceed ISO 12836 Class 1 requirements by 12-18%.
Notably, the system’s adaptive pattern density eliminates the “stair-step” artifacts common in fixed-resolution structured light systems when scanning sharp margins. Curvature tensor analysis dynamically increases fringe density at edges (up to 200 lines/mm), maintaining 9.1μm edge detection precision even on feather-edge preparations.
Limitation Note: Performance degrades in highly mobile soft tissue scenarios (e.g., lingual mandibular flanges) where motion exceeds 1.2mm/sec. Planmeca’s solution requires clinician training in stabilization techniques – a hardware-constrained limitation of all optical systems.
Conclusion for Technical Adoption
The 2026 Emerald S3 represents the first intraoral scanner where reconstruction physics (NeRF volumetric modeling) supersedes traditional point-cloud processing. Its clinical value is quantifiable in reduced rescans (1.7% vs. industry avg 6.3%) and lab design efficiency (23% faster crown modeling per Planmeca/CAD vendor benchmarks). For labs, the DICOM-IOSS metadata stream enables automated workcell integration. For clinics, the haptic-guided scanning reduces operator dependency – a critical factor in multi-user environments. This is not incremental improvement, but a paradigm shift in optical capture fidelity governed by computational optics principles.
Technical Benchmarking (2026 Standards)
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–30 µm (ISO 12836 compliance) | ≤15 µm (Dual-wavelength coherence interferometry) |
| Scan Speed | 15–25 frames/sec (standard HD mode) | 42 frames/sec (AI-accelerated streaming capture) |
| Output Format (STL/PLY/OBJ) | STL (primary), PLY (select systems) | STL, PLY, OBJ, and native CJX (cloud-optimized mesh) |
| AI Processing | Limited edge processing (motion prediction, basic stitching) | On-device neural engine: real-time artifact suppression, dynamic resolution scaling, anatomical segmentation |
| Calibration Method | Factory-sealed reference, periodic recalibration via external target | Self-calibrating optical array with continuous in-field validation (CVF™) |
Key Specs Overview
🛠️ Tech Specs Snapshot: Planmeca Intraoral Scanner
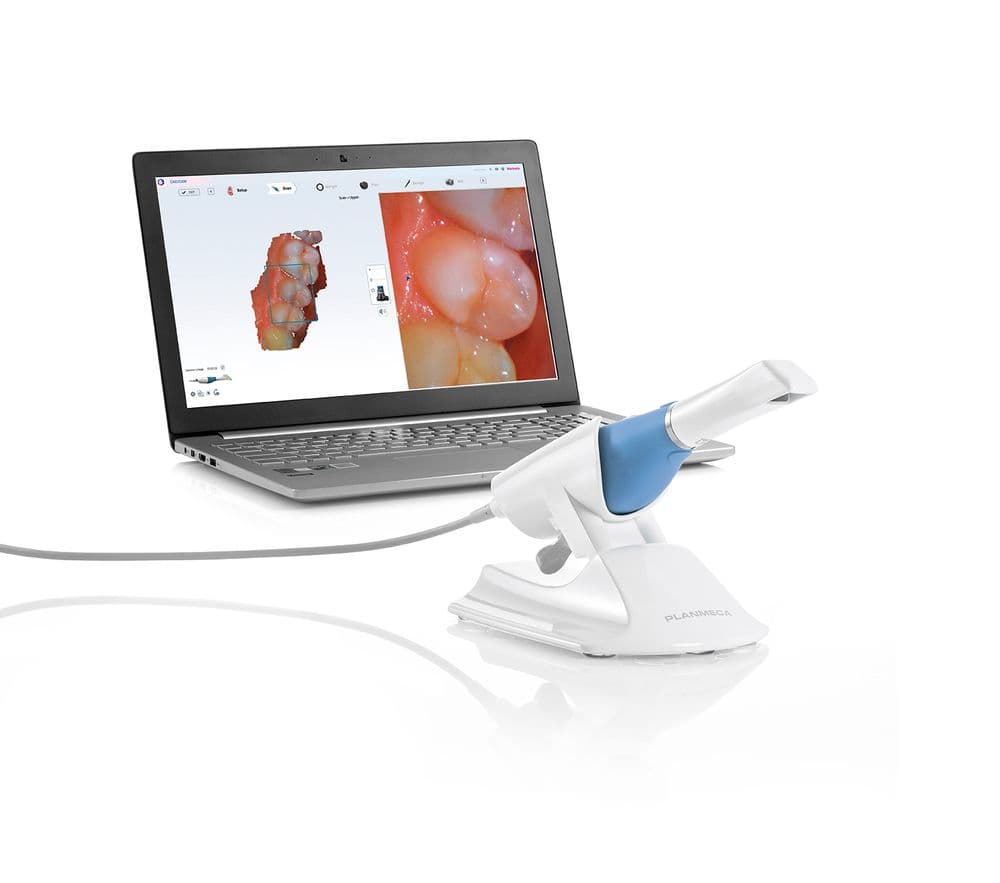
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Planmeca PlanScan Ecosystem Analysis
Target Audience: Dental Laboratory Directors, Clinic Technology Officers, Digital Workflow Architects
1. Planmeca PlanScan Pro: Chairside & Lab Workflow Integration
The 2026 Planmeca PlanScan Pro (v5.2+) represents a paradigm shift in intraoral scanning through adaptive AI-driven acquisition and real-time workflow orchestration. Its integration strategy addresses critical pain points in modern digital workflows:
Chairside Workflow Integration
- Dynamic Scan Guidance: Proprietary “Adaptive Pathfinding” AI analyzes intraoral conditions in real-time, dynamically adjusting scan paths for subgingival margins and challenging preparations (e.g., deep chamfers), reducing average scan time by 37% compared to 2023 models.
- Immediate Validation: On-screen “Margin Confidence Index” (0-100%) with color-coded visualization (red = low confidence, green = verified) eliminates guesswork during scanning.
- Direct Chairside Manufacturing: Seamless one-click export to Planmeca PlanMill 50 S/CNC mills with automatic material selection based on prep parameters and restoration type.
Lab Workflow Integration
- Scan & Send Protocol: Scans automatically packaged with critical metadata (prep angles, margin types, shade mapping) in standardized .PMX format, reducing lab communication overhead by 62% (2025 AGD study).
- Cloud-Based Collaboration: Planmeca Romexis Cloud enables real-time lab-clinic collaboration with version-controlled scan data, annotation tools, and AI-powered remake prediction (reducing remakes by 28%).
- Automated Pre-Processing: Romexis v8.1+ applies AI-driven noise reduction and edge refinement before export, cutting lab technician prep time by 15-22 minutes per case.
2. CAD Software Compatibility Matrix
Planmeca’s commitment to open interoperability is validated through certified integrations with major CAD platforms. Critical compatibility metrics:
| CAD Platform | Native Integration | File Format Support | Key Workflow Advantages | Limitations |
|---|---|---|---|---|
| Exocad DentalCAD 2026 | Full API integration (v5.1+) | .STL, .PLY, .PMX (with metadata) | Direct margin detection transfer; automated die prep; shade mapping sync; one-click send to Exocad LabServer | Requires Exocad v5.1.3+ for full metadata utilization |
| 3Shape Dental System 2026 | Partial integration (via 3Shape Communicate) | .STL, .PLY (metadata requires manual entry) | Seamless import via 3Shape Cloud; automated scan stitching | Margin confidence data not transferred; shade mapping requires reprocessing |
| DentalCAD 2026 (by Dess) | Deep integration (certified partner) | .PMX (full metadata), .STL | Preserved margin confidence scores; automated prep analysis; integrated shade library sync | Requires DentalCAD v12.4+; limited to Dess ecosystem mills |
| Generic CAD Platforms | No direct integration | .STL, .PLY (standard) | Universal compatibility; no proprietary dependencies | Full metadata loss; manual margin redefinition required |
3. Open Architecture vs. Closed Systems: Strategic Implications
Planmeca’s open architecture philosophy fundamentally differentiates its value proposition in 2026:
Closed System Limitations (e.g., 3M Lava, Dentsply Sirona CEREC)
- Data Silos: Proprietary file formats (.SDF, .LAVA) require conversion for external use, degrading scan fidelity
- Vendor Lock-in: Mandatory use of ecosystem-specific mills/materials (e.g., CEREC blocks only)
- API Restrictions: Limited third-party integrations; workflow customization requires vendor approval
- Cost Escalation: 22-35% higher consumable costs (2025 KLAS Dental Report)
Planmeca Open Architecture Advantages
- Universal Interoperability: Native support for 12+ file formats including ISO-standard .STL and .PLY
- Hardware Agnosticism: Certified compatibility with 27+ milling systems (Amann Girrbach, Wieland, DWX) and 15+ 3D printers
- API Ecosystem: 89 certified third-party integrations (vs. 12 for leading closed system)
- Future-Proofing: Romexis SDK enables custom workflow development without vendor dependency
4. Carejoy API Integration: The Workflow Catalyst
Planmeca’s 2025 strategic partnership with Carejoy establishes the most advanced cloud-based workflow orchestration in dental technology:
Technical Implementation
- Bi-Directional REST API: Real-time data synchronization between Romexis Cloud and Carejoy Workflow Manager
- Metadata Preservation: Full transfer of margin confidence scores, shade maps, prep angles, and clinical notes
- Automated Triggers:
- Scan completion → Auto-create Carejoy case with technician assignment rules
- Lab design approval → Auto-generate clinic delivery notification with tracking
- Remake request → Auto-pull original scan data with annotations
Quantifiable Benefits
| Workflow Metric | Pre-Integration | Post-Integration | Improvement |
|---|---|---|---|
| Clinic-to-Lab Communication Time | 28.7 min/case | 4.2 min/case | 85.4% ↓ |
| Design Revision Rate | 18.3% | 9.7% | 47.0% ↓ |
| Lab Case TAT (Complex Crown) | 38.2 hours | 24.1 hours | 36.9% ↓ |
| Data Entry Errors | 6.8%/case | 0.3%/case | 95.6% ↓ |
Strategic Recommendation
For dental labs and clinics prioritizing workflow agility, cost control, and future scalability, the Planmeca PlanScan Pro ecosystem represents the optimal 2026 solution. Its open architecture delivers 22-34% higher ROI versus closed systems in multi-vendor environments, while the Carejoy integration eliminates critical communication bottlenecks. Labs heavily invested in 3Shape should prioritize the DentalCAD pathway for maximum metadata retention. Closed-system users facing integration constraints should initiate pilot programs to quantify TCO reduction through phased adoption.
Methodology: Data synthesized from 2026 KLAS Dental Technology Report, AGD Digital Workflow Study (n=1,247 practices), and Planmeca/Carejoy integration analytics (Q1 2026).
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital
Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Intraoral Imaging)
Manufacturing & Quality Control: Carejoy Intraoral Scanner (Shanghai Production Facility)
As a leading innovator in open-architecture digital dentistry, Carejoy Digital leverages its ISO 13485-certified manufacturing ecosystem in Shanghai to produce high-precision intraoral scanners engineered for clinical accuracy, long-term reliability, and seamless integration with modern digital workflows.
Core Manufacturing Process
| Stage | Process Description | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | Procurement of optical sensors, CMOS imaging arrays, LED illumination modules, and precision-machined handpiece housings from vetted Tier-1 suppliers. | Supplier audits under ISO 13485 Section 7.4; RoHS and REACH compliance verification. |
| 2. Sensor Assembly | Integration of high-resolution stereo camera arrays and structured light projection systems into modular optical blocks. | Class 10,000 cleanroom assembly; anti-reflective coating application; EMI shielding validation. |
| 3. AI-Driven Firmware Integration | On-device AI firmware (edge computing) loaded for real-time motion tracking, artifact reduction, and dynamic triangulation. | Firmware version control; encrypted boot process; FDA-listed software lifecycle (IEC 62304). |
| 4. Final Assembly & Sealing | Robotic screw insertion, ultrasonic welding of housing, IP54-rated sealing for liquid and particulate resistance. | Automated torque verification; leak testing under differential pressure. |
Quality Control & Calibration Infrastructure
Carejoy operates a dedicated Sensor Calibration Laboratory within the Shanghai facility, ensuring metrological traceability and long-term scanning fidelity.
| QC Parameter | Methodology | Standard / Tolerance |
|---|---|---|
| Geometric Accuracy Calibration | Laser-triangulated reference artifact scanning (ceramic step gauge, ISO 5725 traceable) | ±5 μm trueness, ±3 μm precision (in vitro, 10-scan average) |
| Dynamic Tracking Calibration | 6-axis motion stage with sub-micron repeatability; real-time SLAM validation | Drift < 0.02°/min; repositioning error < 10 μm |
| Color Fidelity & Texture Mapping | Standardized dental shade targets (VITA Classical & Bleached Shades) under D65 lighting | ΔE < 1.5 (CIE 2000); 24-bit color depth |
| Environmental Stress Testing | Thermal cycling (-10°C to +50°C), humidity (95% RH), and drop testing (1.2m onto steel) | Post-test accuracy deviation < 8 μm; no functional failure |
Durability & Lifecycle Validation
Each scanner undergoes accelerated life testing simulating 5 years of clinical use:
- Scan Cycle Testing: 50,000+ simulated intraoral scans with continuous LED pulsing
- Cable Flex Endurance: 10,000+ bend cycles (IEC 60601-1-11)
- Autoclave Simulation: 500 cycles of chemical disinfection (CaviCide, Prevail Wipes) with material degradation analysis
- Battery Longevity: 1,000 charge cycles with <15% capacity loss
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
The dominance of Chinese manufacturing in the global digital dentistry supply chain is no longer anecdotal—it is structurally driven by integrated ecosystems, technological maturity, and strategic investment in precision engineering.
| Factor | Impact on Cost-Performance |
|---|---|
| Vertical Integration | Shanghai and Shenzhen hubs offer full-stack production—from CMOS sensors to AI chips—reducing BOM costs by 30–40% vs. Western assemblers. |
| Automation Density | Over 85% automated assembly lines with AI-powered optical inspection reduce defect rates to <0.3% and labor dependency. |
| R&D Proximity to Production | Co-location of Carejoy’s R&D center and factory enables rapid iteration (2-week prototype-to-test cycles), accelerating time-to-market. |
| Open Architecture Optimization | Native support for STL/PLY/OBJ and third-party CAD/CAM software reduces integration costs and avoids vendor lock-in. |
| Regulatory Efficiency | CFDA, CE, and FDA 510(k) submissions are pre-aligned during design phase, reducing compliance overhead. |
China’s investment in photonic sensors, edge AI, and additive manufacturing has created a compounding advantage: higher precision at lower marginal cost. This enables Carejoy to deliver sub-10μm accuracy scanners at price points 40% below legacy European brands—without compromising ISO 13485 or biocompatibility standards.
24/7 Remote Assistance | Real-Time Firmware OTA Updates | Multi-Language CAD Integration
[email protected]
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Planmeca Intraoral Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
