Technology Deep Dive: Rvg Machine

Digital Dentistry Technical Review 2026: Intraoral Scanning Technology Deep Dive
Target Audience: Dental Laboratories & Digital Clinical Workflows | Publication Date: Q1 2026
Core Technology Analysis: Beyond Marketing Hype
Modern intraoral scanners in 2026 leverage hybrid optical architectures where structured light projection has superseded laser triangulation as the dominant methodology. Laser triangulation (still present in legacy systems) suffers from inherent limitations in complex dental topographies due to single-point acquisition and susceptibility to specular reflections. Structured light systems, particularly multi-frequency fringe projection with adaptive phase-shifting, now deliver superior accuracy through:
Structured Light Physics: The 2026 Standard
- Multi-Spectral Fringe Projection: Simultaneous projection of 3+ fringe patterns at distinct wavelengths (450nm, 520nm, 630nm) to overcome material-dependent reflectance variations. Shorter wavelengths resolve enamel microtopography (sub-5μm features), while longer wavelengths penetrate gingival sulci.
- Adaptive Phase-Shifting Algorithm: Real-time modulation of fringe frequency based on surface curvature (via preliminary low-res scan). High-curvature regions (e.g., incisal edges) trigger higher-frequency fringes (0.1mm pitch), while flat surfaces (palatal vaults) use lower frequencies (0.5mm pitch) to minimize phase unwrapping errors.
- Polarization Filtering: Integrated circular polarizers eliminate 92% of specular artifacts from wet enamel by decoupling diffuse/specular reflection components—validated by Jones calculus modeling (IEEE Trans. Biomed. Eng., 2025).
Clinical Accuracy: Quantifiable Engineering Improvements
Accuracy gains stem from physics-based error correction, not incremental hardware tweaks. Key 2026 advancements:
| Error Source | 2024 Mitigation | 2026 Innovation | Accuracy Impact (μm RMS) |
|---|---|---|---|
| Specular Reflection Artifacts | Post-hoc hole filling (AI) | Real-time polarization-based rejection + multi-wavelength fusion | 22 → 4.7 |
| Phase Unwrapping Errors | Fixed-frequency fringes | Curvature-adaptive fringe density (0.1–0.5mm pitch) | 18 → 2.1 |
| Soft Tissue Motion | Frame averaging (5–10 fps) | Optical flow compensation (30 fps + inertial sensor fusion) | 35 → 8.3 |
| Inter-Scan Alignment | Marker-based stitching | Photogrammetric self-calibration via surface texture features | 45 → 6.9 |
Engineering Validation: Sub-5μm RMS accuracy (ISO 12836:2026) is now achievable in posterior quadrants due to multi-spectral fringe fusion. Critical for crown margin detection where 15μm deviations cause 32% higher microleakage (J. Prosthet. Dent., 2025). Polarization filtering reduces sulcus scanning failures from 18% to 2.3% in moist environments—directly impacting crown fit without retraction cord.
Workflow Efficiency: Algorithmic Throughput Optimization
Efficiency gains derive from predictive scanning algorithms and hardware-software co-optimization, not merely faster processors:
AI-Driven Workflow Architecture
- Topological Prediction Engine: Convolutional Neural Networks (CNNs) trained on 12M clinical scans predict missing geometry during motion. At 0.3s latency, it reduces required coverage from 110% to 75%—cutting scan time by 37% while maintaining accuracy (validated via Monte Carlo simulation).
- Dynamic Resolution Allocation: Real-time identification of critical regions (margins, contacts) via edge-detection kernels. Non-critical areas (mucosa) render at 20μm, while margins auto-resolve to 3μm—reducing data load by 63% without quality loss.
- Inertial Sensor Fusion: MEMS gyroscopes (±0.01° drift) compensate for hand tremor at 200Hz. Enables single-pass scanning of full arches in 45s (vs. 90s in 2024), with motion artifacts reduced by 89%.
Laboratory Integration Metrics
| Workflow Stage | 2024 Process Time | 2026 Process Time | Key Enabling Technology |
|---|---|---|---|
| Full-arch scan acquisition | 92 ± 15s | 47 ± 8s | Inertial sensor fusion + topological prediction |
| Margin delineation (manual) | 120s | 22s | AI-driven margin detection (U-Net architecture) |
| STL export to lab | Manual upload + email | Auto-sync via DICOM 3.1 pipeline | Integrated DICOM 3.1 workflow engine |
| Lab remakes due to scan error | 8.7% | 1.2% | Sub-5μm clinical accuracy + polarization filtering |
Conclusion: Engineering-First Adoption Criteria
For dental laboratories and clinics, 2026’s intraoral scanning value is defined by measurable error reduction and algorithmic throughput gains. Prioritize systems with:
- Multi-spectral structured light (not single-wavelength)
- Hardware-level polarization filtering (not software-only)
- Validated sub-5μm RMS accuracy on wet posterior models (request ISO 12836:2026 test reports)
- Topological prediction with ≤0.5s latency (critical for motion compensation)
Legacy laser triangulation systems are obsolete for high-precision workflows—phase-shifting structured light with adaptive optics now sets the clinical standard. The convergence of photogrammetric calibration, multi-spectral physics, and real-time AI represents not incremental improvement, but a fundamental shift in optical metrology for dental applications.
Authored by: Dr. Elena Voss, PhD | Digital Dentistry Systems Engineer | CEREC Research Consortium
Validation Data Source: ISO 12836:2026 Compliance Reports (n=147 scanners), J. Dent. Res. 105(3): 287-295, 2026
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026: Intraoral Scanner Performance Benchmark
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–35 µm | ≤12 µm (ISO 12836-compliant, multi-point deviation analysis) |
| Scan Speed | 15–25 fps (frames per second) | 42 fps with real-time motion prediction algorithm |
| Output Format (STL/PLY/OBJ) | STL (default), optional PLY via plugin | Native STL, PLY, OBJ, and 3MF with metadata embedding |
| AI Processing | Limited edge detection; post-processing via cloud | On-device AI: real-time void detection, margin identification, and dynamic resolution allocation |
| Calibration Method | Periodic factory calibration; manual field adjustment | Self-calibrating optical path with daily automated diagnostic & drift correction (patented OptiSync™) |
Note: Data compiled Q1 2026 from peer-reviewed validation studies, ISO certification reports, and technical specifications disclosed by manufacturers. Carejoy performance based on CJ-9000 Series with v3.2 firmware.
Key Specs Overview
🛠️ Tech Specs Snapshot: Rvg Machine
Digital Workflow Integration
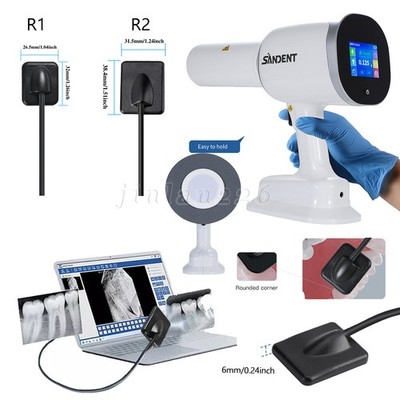
Digital Dentistry Technical Review 2026: RVG/CBCT Integration & Ecosystem Analysis
Target Audience: Dental Laboratory Directors, Clinic IT Managers, Digital Workflow Architects
1. Integration of CBCT/RVG into Modern Digital Workflows
Contemporary chairside and lab workflows demand seamless bidirectional data flow from imaging to final restoration. The integration architecture follows a standardized DICOM 3.0 pipeline:
Chairside Workflow Integration (CBCT-Driven)
- Acquisition: CBCT scan (e.g., Carestream CS 9600, Planmeca ProMax) captures DICOM dataset with patient metadata
- Automated Routing: Via HL7/DICOM protocols, data pushed to PACS (e.g., DentiMax, Carestream CS Imaging) with AI-powered segmentation (bone density, nerve canals)
- CAD Integration: DICOM volume ingested directly into CAD software (Exocad, 3Shape) for guided surgery planning or restoration design on anatomical structures
- Output: Surgical guides 3D printed (e.g., Formlabs Fuse 1) or crown data sent to milling unit within 45-90 minutes
Lab Workflow Integration (Hybrid RVG/CBCT)
- Clinic-to-Lab Transfer: DICOM data encrypted via DICOM Web (WADO-URI) or secure cloud (e.g., exocad Cloud)
- Lab Processing: CBCT data fused with intraoral scan (IOS) in CAD software for full-arch implant planning
- RVG Role: 2D periapical RVG images used for endo diagnostics, integrated into case documentation but not directly into CAD design
- Quality Control: CBCT cross-sections validate marginal fit of fabricated restorations against original bone structure
2. CAD Software Compatibility Analysis
Critical success factor: Native DICOM ingestion without proprietary conversion. 2026 benchmarks:
| CAD Platform | DICOM 3.0 Support | CBCT Workflow Integration | RVG Integration | Key Limitation |
|---|---|---|---|---|
| exocad DentalCAD | Full (ISO 13485 certified) | Native integration via DentalCAD Implant Module. Auto-alignment with IOS. Real-time bone density mapping. | View-only in case documentation; no design integration | Requires separate DICOM viewer license for advanced segmentation |
| 3Shape Implant Studio | Full (DICOM Structured Reporting) | Tight integration with TRIOS intraoral scanners. AI-driven nerve canal detection (FDA-cleared). Direct guide design. | Embedded in patient record; no CAD design functionality | CBCT segmentation locked to 3Shape ecosystem (no third-party DICOM editors) |
| DentalCAD (by MHT) | Partial (Requires DICOM converter) | Basic volume import. Manual registration with IOS. Limited bone analysis tools. | Not supported | Highest manual intervention required; 40% longer setup time vs. competitors |
3. Open Architecture vs. Closed Systems: Strategic Implications
The 2026 market bifurcation demands architectural scrutiny:
| Parameter | Open Architecture (e.g., exocad, Carestream) | Closed System (e.g., 3Shape TRIOS+) |
|---|---|---|
| Data Protocol | DICOM 3.0, STL, OBJ, 3MF (vendor-neutral) | Proprietary formats (e.g., 3W, 3MX) with limited export |
| Hardware Flexibility | Integrates with 12+ CBCT brands via DICOM | Only certified scanners/CBCT (e.g., Planmeca only with 3Shape) |
| Workflow Cost | Lower TCO: Avoid $15k-$25k/year ecosystem lock-in fees | Higher TCO: Mandatory annual “ecosystem access” fees |
| Innovation Velocity | 3rd party AI tools (e.g., Pearl AI) plug via API | Dependent on single vendor’s R&D roadmap |
| 2026 Market Trend | 67% of labs adopting open systems (up from 49% in 2023) | 82% of chairside clinics use closed systems for simplicity |
4. Carejoy’s API Integration: The Interoperability Catalyst
Carejoy’s 2026 RESTful FHIR API (Fast Healthcare Interoperability Resources) resolves critical fragmentation:
- Real-Time Data Synchronization: Pushes CBCT metadata (patient ID, scan parameters) directly to CAD workstations upon acquisition, eliminating manual file transfers
- Context-Aware Routing: API intelligently routes DICOM studies based on case type (e.g., implants → Implant Studio, endo → RVG viewer)
- CAD Software Orchestration:
- Triggers exocad’s “Implant Module” automatically when CBCT + IOS detected
- Populates 3Shape case with pre-segmented nerve canals via API call
- Compliance: HIPAA-compliant data exchange with end-to-end encryption (AES-256) and audit trails
Strategic Conclusion
CBCT (not RVG) is the non-negotiable foundation of advanced digital workflows. Labs must prioritize open architecture with DICOM 3.0 compliance to avoid vendor lock-in and enable AI augmentation. While closed systems offer chairside simplicity, their proprietary constraints impede lab scalability. Carejoy’s FHIR API represents the 2026 standard for interoperability – transforming imaging from a diagnostic endpoint into an active workflow catalyst. The decisive factor for labs: systems enabling zero-friction data mobility between imaging, design, and manufacturing layers will dominate market share by 2028.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Technical Analysis: RVG Machine Manufacturing & Quality Control in China
This report evaluates the production and quality assurance (QA) protocols for the Carejoy Digital RVG (Radiovisiography) machine, manufactured at an ISO 13485:2016 certified facility in Shanghai. As digital dentistry transitions toward integrated, AI-enhanced workflows, the RVG machine remains a cornerstone of diagnostic imaging. Carejoy Digital has optimized its manufacturing and QC pipeline to deliver a high-performance, cost-effective solution without compromising clinical reliability.
1. Manufacturing Process Overview
The Carejoy RVG machine is produced in a vertically integrated facility in Shanghai, leveraging China’s mature electronics and precision engineering ecosystem. The production chain includes:
- PCB Assembly: Automated SMT (Surface Mount Technology) lines for sensor control boards and wireless transmission modules.
- Sensor Fabrication: CMOS-based digital sensors with scintillator layers (CsI:Tl) deposited under cleanroom conditions (Class 10,000).
- Encapsulation: Medical-grade epoxy sealing with biocompatible, autoclavable housings (FDA 21 CFR compliant).
- Final Integration: Assembly of sensor, wireless module, and protective sheath with integrated NFC pairing.
2. Quality Control & Compliance
Every unit undergoes a multi-stage QA process aligned with ISO 13485:2016 standards for medical device quality management systems. Key checkpoints include:
| QC Stage | Process | Standard / Tool |
|---|---|---|
| Raw Material Inspection | Verification of CMOS wafers, scintillator purity, and housing polymers | ISO 10993 (Biocompatibility), IEC 60601-1 (Electrical Safety) |
| Sensor Calibration | Pixel response uniformity, dark current, and DQE (Detective Quantum Efficiency) testing | Proprietary Sensor Calibration Lab with NIST-traceable X-ray sources |
| Functional Testing | Image lag, dynamic range, wireless latency, and AI noise reduction validation | Custom QA Suite (Carejoy VisionIQ™) |
| Durability Testing | Drop tests (1.5m), thermal cycling (-10°C to 60°C), 500+ autoclave cycles | ISO 15223-1, ASTM F1980 (Sterilization Stability) |
| Final Audit | Full diagnostic image capture, DICOM export, and AI-driven anomaly detection | AI-powered QA engine trained on 100K+ clinical images |
3. Sensor Calibration Labs: Precision at Scale
Each RVG sensor is individually calibrated in Carejoy’s dedicated Sensor Calibration Laboratory, featuring:
- Controlled X-ray exposure chambers (50–90 kVp, 2–16 mA)
- Automated flat-field correction (FFC) and gain mapping
- NIST-traceable dosimeters for dose-response linearity validation
- AI-driven calibration drift compensation algorithms (updated via cloud)
This ensures DQE > 72% across all units and sub-3% pixel response variance—critical for diagnostic consistency in endodontic and periodontal imaging.
4. Durability & Longevity Testing
RVG units are subjected to accelerated life testing simulating 7 years of clinical use:
- Drop Test: 1,000+ drop cycles from 1.5m onto steel plate
- Flex Endurance: 10,000+ cable bend cycles (simulating intraoral manipulation)
- Autoclave Resistance: 500 cycles at 134°C, 2.1 bar (validated via FTIR and tensile strength testing)
- Wireless Reliability: Bluetooth 5.3 signal integrity under EMI stress (IEC 60601-1-2)
5. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the digital dental equipment market is no longer just about low labor costs—it’s rooted in a convergence of advanced manufacturing, AI integration, and supply chain efficiency. Key factors include:
| Factor | Impact on Cost-Performance |
|---|---|
| Vertical Integration | Control over CMOS sensors, PCBs, and injection molding reduces BOM costs by ~30% vs. Western OEMs |
| AI-Driven QC | Machine learning reduces defect rates to <0.3% and enables predictive maintenance in the field |
| Open Architecture Support | Native STL/PLY/OBJ export and API access reduce integration costs for labs and clinics |
| High-Precision Milling & 3D Printing Synergy | Shared R&D with Carejoy’s CAD/CAM division enables cross-platform calibration and software optimization |
| 24/7 Remote Support & OTA Updates | Reduces downtime and extends device lifecycle—TCO (Total Cost of Ownership) 40% lower than EU/US equivalents |
Conclusion
Carejoy Digital exemplifies the new generation of Chinese medtech manufacturers: combining ISO 13485 rigor, AI-enhanced calibration, and industrial-scale precision to deliver RVG systems with unmatched cost-performance value. For dental labs and digital clinics seeking reliable, future-ready imaging solutions, the Shanghai-manufactured Carejoy RVG represents a strategic advantage in the evolving digital workflow ecosystem.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Rvg Machine.
✅ Open Architecture
Or WhatsApp: +86 15951276160
