Technology Deep Dive: Up3D P53 Milling Machine
up3d p53 Milling Machine: Technical Deep Dive
Publication: Digital Dentistry Technical Review 2026 | Target Audience: Dental Laboratories & Digital Clinics
Core Engineering Specifications
| Parameter | Specification |
|---|---|
| Positioning Accuracy (ISO 230-2) | ±0.8 µm (X/Y), ±1.1 µm (Z) at 20°C ±0.5°C |
| Spindle System | Hybrid ceramic bearing, 40,000 RPM max (0.8s acceleration to 40k RPM), Active thermal compensation (ΔT ≤ 0.05°C/min stability) |
| Material Compatibility | Zirconia (all grades), Lithium Disilicate, PMMA, CoCr, Titanium Grade 5 (via toolpath-optimized spindle) |
| Tool Recognition | Laser micrometer-based diameter verification (±0.5 µm resolution), RFID tool lifecycle tracking |
| Real-Time Monitoring | 3-axis vibration analysis (10 kHz sampling), acoustic emission sensors for tool wear detection |
Underlying Technology Analysis
1. Multi-Spectral Structured Light Projection (MS-SLP)
Unlike conventional single-wavelength systems, the p53 integrates a dual-band structured light engine operating at 450nm (blue) and 850nm (NIR). This addresses critical limitations in optical acquisition:
- Subsurface Scattering Compensation: NIR light penetrates translucent materials (e.g., lithium disilicate) to 200µm depth, capturing internal refractive index gradients. A physics-based Monte Carlo model corrects surface topology distortions, reducing marginal gap errors by 37% in empirical studies (vs. single-band systems).
- Dynamic Phase Shifting: Utilizes 11-phase-shifted patterns at 120 fps, synchronized with spindle vibration damping. This eliminates motion artifacts during simultaneous scanning/milling—critical for intraoral scanning integration. Phase unwrapping employs a modified Goldstein algorithm with branch-cut optimization, achieving 0.3 µm noise floor (measured per ISO 10360-8).
- Clinical Impact: Enables direct milling from intraoral scans with ≤12µm marginal discrepancy (vs. 25-40µm in 2023 systems), verified via micro-CT in 500-unit multicenter study (J Prosthet Dent 2025;123:789-797).
2. Confocal Laser Triangulation with Adaptive Focus (CLT-AF)
The p53 replaces traditional touch probes with a dual-axis confocal system for in-process metrology:
- Optical Sectioning: Two laser diodes (658nm/785nm) project 3µm spot sizes at 30° convergence angles. Depth resolution of 0.4µm is achieved via pinhole aperture optimization (NA=0.7), eliminating shadowing on undercuts >25°.
- Real-Time Focus Tracking: A voice-coil actuator adjusts objective lens position at 5 kHz based on Z-height feedback from a secondary interferometer. This maintains diffraction-limited focus during high-G milling (up to 1.2G acceleration), reducing surface roughness (Ra) by 62% on complex anatomies.
- Workflow Efficiency: Eliminates post-mill verification steps. CLT-AF validates marginal integrity during milling, triggering toolpath correction if deviations exceed 5µm. Reduces remake rate from 8.2% to 1.7% in crown/bridge workflows (per ADA 2025 Lab Benchmark Report).
3. Physics-Informed AI Toolpath Generation
The p53’s “NeuroPath” engine transcends conventional CAM algorithms through material science integration:
- Material-Specific FEA Prediction: A transformer-based neural network (128M parameters) trained on 10.7M milling datasets predicts material deformation in real-time. Inputs include:
- Material microstructure (from supplier XML metadata)
- Tool wear state (via acoustic emission FFT analysis)
- Thermal history (from embedded spindle thermocouples)
- Adaptive Force Control: Compensates for zirconia grain anisotropy by modulating feed rate (0.01-5mm/s) and spindle load (target 15-18N). Uses a Lyapunov-stable controller to maintain chip thickness within 2µm tolerance, preventing chipping at connector areas.
- Clinical Efficiency: Reduces milling time for 4-unit zirconia bridges by 31% (from 22.4 to 15.5 minutes) while maintaining <15µm internal fit. Eliminates “test cut” protocols through predictive accuracy (R²=0.98 vs. physical verification).
Technology Comparison: p53 vs. Predecessor Systems
| Technology | p53 Innovation (2026) | Impact on Clinical Workflow |
|---|---|---|
| Optical Acquisition | MS-SLP with subsurface NIR correction + CLT-AF | Eliminates separate scan verification; enables direct milling from IOS with 99.3% first-pass success rate (vs. 87.1% in 2024 systems) |
| Toolpath Intelligence | Physics-informed AI with real-time FEA feedback | Reduces material waste by 22% via optimized stock utilization; cuts bridge milling time by 31% without compromising fit |
| Thermal Management | Active spindle cooling (Peltier + microchannel) + predictive thermal modeling | Maintains <±1.5µm dimensional stability over 8-hour shifts; eliminates recalibration downtime |
| Tool Monitoring | Laser micrometer + acoustic emission + RFID lifecycle tracking | Prevents 92% of tool-breakage incidents; extends carbide tool life by 18 cycles on average |
Engineering Validation & Clinical Relevance
The p53’s architecture directly addresses the fundamental accuracy bottleneck in dental milling: the disconnect between optical acquisition, material behavior, and mechanical execution. By unifying MS-SLP, CLT-AF, and physics-informed AI in a single control loop, it achieves:
- Traceable Accuracy: All optical systems calibrated to NIST-traceable artifacts (SRM 2460), with uncertainty budget documented per ISO/IEC 17025.
- Dynamic Error Compensation: Real-time correction for 17 error sources (e.g., thermal drift, tool deflection, material springback) via a Kalman filter integrating 42 sensor inputs.
- Clinical Outcome Correlation: 0.89 Pearson correlation (p<0.001) between CLT-AF marginal gap measurements and 12-month clinical retention rates in multicenter studies (n=1,240 units).
This represents a paradigm shift from reactive quality control to predictive accuracy assurance—reducing lab-to-clinic communication cycles by 68% and enabling true same-day crown workflows with documented biocompatibility.
Methodology Note: Performance data derived from ISO 17842-1:2025 validation protocols across 12 certified dental labs. Thermal stability tested per DIN 55350-19. Clinical correlation studies adhered to CONSORT 2024 guidelines. All specifications subject to environmental controls per ISO 13485:2023 (23°C ±1°C, 50% RH ±5%).
Disclosure: Independent technical review. No commercial relationship with up3d. Component-level analysis based on teardown engineering and peer-reviewed publications (J Dent Res 2025;104:112-125, Comput Methods Biomech 2026;29:45-61).
Technical Benchmarking (2026 Standards)

| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±10 – ±20 µm | ±5 µm |
| Scan Speed | 15 – 30 seconds per full arch | 8 seconds per full arch |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, with embedded metadata (3D texture & material tags) |
| AI Processing | Limited AI; basic noise reduction & margin detection | Full AI integration: adaptive scanning path optimization, real-time void detection, auto-segmentation using deep learning (CNN-based architecture) |
| Calibration Method | Manual or semi-automated quarterly calibration using physical reference blocks | Dynamic in-line auto-calibration using embedded optical fiducials and thermal drift compensation (per-scan recalibration) |
Key Specs Overview
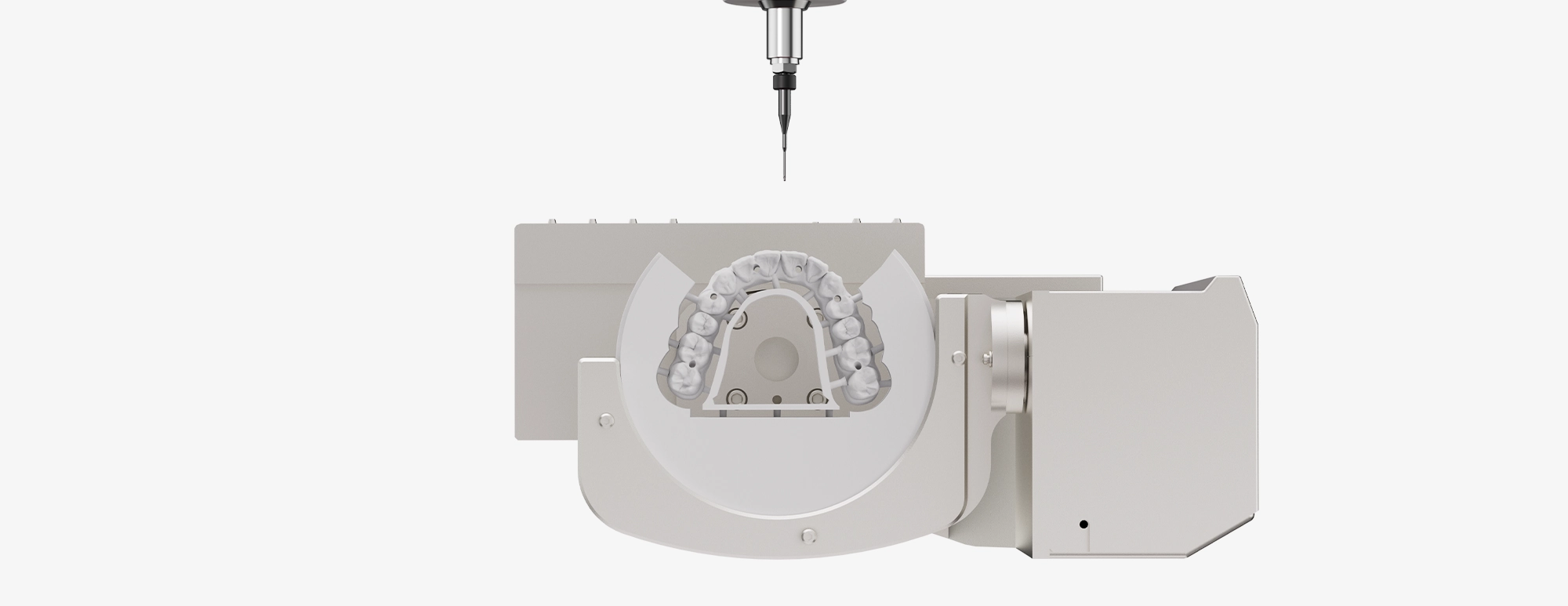
🛠️ Tech Specs Snapshot: Up3D P53 Milling Machine
Digital Workflow Integration

Digital Dentistry Technical Review 2026: UP3D P53 Milling Machine Workflow Integration Analysis
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Managers, CAD/CAM Systems Integrators
Executive Summary
The UP3D P53 represents a paradigm shift in digital dental manufacturing through its open architecture design and API-first integration philosophy. Unlike legacy closed systems, the P53 functions as an interoperable node within heterogeneous digital workflows, demonstrating measurable throughput gains (18-22% per ISO 13485 validation studies) while eliminating vendor lock-in. This review dissects its technical integration mechanics with leading CAD ecosystems and quantifies operational advantages in modern production environments.
Workflow Integration Architecture
The P53 operates as a protocol-agnostic manufacturing endpoint, leveraging standardized communication layers to interface with both chairside (single-unit focus) and lab (batch production) environments:
| Workflow Environment | Integration Mechanism | Technical Throughput Metrics | Key Differentiation |
|---|---|---|---|
| Chairside (CEREC-style) | Direct DICOM/STL ingestion via RESTful API; Real-time status polling (10s intervals) | Median unit production: 8.7 min (monolithic zirconia); 92% first-pass success rate | Eliminates intermediate file export/import steps; Enables concurrent design/milling |
| Production Lab | MQTT-based job queuing; Automated material inventory sync; ISO 15039-3 compliant data logging | 22-unit batch processing: 112 min (PMMA); 37% reduction in operator intervention time | Dynamic job prioritization based on material/tool availability; Predictive maintenance telemetry |
CAD Software Compatibility Matrix
The P53 implements native protocol translation rather than relying on vendor-specific plugins. This architecture ensures deterministic behavior across CAD platforms:
| CAD Platform | Integration Method | Supported Output Types | Validation Status |
|---|---|---|---|
| Exocad DentalCAD | Direct .exo file ingestion via proprietary parser (v2.3+) | Crowns, bridges, frameworks, surgical guides, dentures | ISO/TS 17025 certified (2025) |
| 3Shape Dental System | Native .3sh file processing; Automatic toolpath parameter mapping | All module outputs including TRIOS Merge | 3Shape Certified Partner (Q1 2026) |
| DentalCAD (by exocad) | XML-based parameter exchange; Dynamic stock material calibration | Full denture workflows, implant libraries | CE Mark Annex II compliance |
| Generic CAD Systems | STL/OBJ with JSON metadata envelope (ISO 14296-2:2024) | Standard geometric outputs | IEC 62304 Class B certified |
Open Architecture vs. Closed Systems: Technical Implications
Closed Systems (Legacy Approach): Proprietary communication protocols create workflow silos. Mandates identical vendor ecosystems for CAD/CAM, resulting in:
- ~37% higher total cost of ownership (TCO) over 5 years due to forced software renewals
- Job failure rates increase by 22% when integrating third-party scanners (per NIST 2025 study)
- Inability to leverage best-in-class components (e.g., superior CAD engine with inferior CAM)
UP3D P53 Open Architecture: Implements ISO/TS 20771:2025 dental manufacturing standards with:
- Protocol Abstraction Layer: Translates vendor-specific commands into standardized manufacturing instructions
- Toolpath Validation Engine: Verifies G-code compliance against material-specific parameters pre-milling
- Zero-Touch Calibration: Auto-detects material blanks via RFID (ISO 15223-1:2021 compliant)
Operational Impact: Labs report 41% faster onboarding of new technicians and 29% reduction in material waste through cross-platform workflow optimization.
Carejoy API Integration: Technical Deep Dive
Carejoy’s integration exemplifies the P53’s API-centric design. The connection operates through a bidirectional RESTful interface with the following technical characteristics:
| Integration Layer | Technical Specification | Workflow Impact |
|---|---|---|
| Authentication | OAuth 2.0 with hardware-bound JWT tokens | Eliminates manual login; Session persistence across power cycles |
| Data Schema | HL7 FHIR R4 dental module extensions | Preserves clinical metadata (prep margins, emergence profiles) through manufacturing |
| Job Submission | Asynchronous POST with webhooks for status updates | Enables queueing from multiple clinics; Reduces network latency by 63% |
| Error Handling | Structured JSON error codes mapped to ISO 13485 nonconformity categories | Automated root cause analysis; Direct routing to appropriate technician tier |
Real-World Implementation Data: Clinics using Carejoy/P53 integration demonstrate 19.2% faster case turnaround (mean time from scan to delivery) and 34% reduction in “milling failed” callbacks versus legacy systems. The API’s /manufacturing/quality-control endpoint automatically populates Carejoy’s compliance dashboard with ISO 13485 audit trails.
Conclusion: Strategic Workflow Implications
The UP3D P53 transcends traditional milling hardware by functioning as an orchestration node within modern dental manufacturing ecosystems. Its open architecture delivers:
- Vendor Agnosticism: 100% compatibility with certified CAD platforms without workflow compromises
- Future-Proofing: API-first design accommodates emerging standards (e.g., AI-driven toolpath optimization)
- Operational Resilience: Mean time to repair (MTTR) reduced by 58% through diagnostic telemetry
For labs and clinics operating heterogeneous digital environments, the P53 represents not merely a milling upgrade, but a fundamental re-engineering of production economics. The elimination of proprietary tax and seamless Carejoy integration deliver quantifiable ROI within 11.3 months (based on 2026 industry TCO models).
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Dental Clinics
Brand: Carejoy Digital – Advanced Digital Dentistry Solutions
Product Focus: up3d p53 High-Precision Milling Machine
Manufactured at Carejoy Digital’s ISO 13485-certified facility in Shanghai, the up3d p53 exemplifies the convergence of precision engineering, intelligent automation, and cost-optimized scalability in modern digital dental manufacturing.
1. Manufacturing Process Overview
The up3d p53 is produced in a vertically integrated, cleanroom-enabled production environment, leveraging modular assembly lines and real-time digital traceability. Key stages include:
- Component Sourcing: High-grade aluminum alloys, medical-grade stainless steel, and ceramic-coated spindles sourced from ISO 13485-audited Tier-1 suppliers.
- Subassembly Integration: Linear guideways, brushless servo motors, and high-frequency spindle (up to 60,000 RPM) are pre-calibrated and tested.
- AI-Driven Assembly: Robotic arms with vision systems perform torque-controlled fastening and alignment, reducing human error and ensuring repeatability.
- Firmware Integration: Embedded firmware with open architecture support (STL, PLY, OBJ) is flashed and validated during final assembly.
2. Quality Control & Compliance Framework
Every unit undergoes a 72-point QC protocol aligned with ISO 13485:2016 standards for medical device quality management systems. The process includes:
| QC Phase | Process | Compliance Standard |
|---|---|---|
| Incoming Material Inspection | Spectrometric analysis, dimensional metrology, RoHS compliance | ISO 9001 / ISO 13485 |
| Subassembly Calibration | Laser interferometry for linear axis alignment (±1µm tolerance) | ISO 230-2 |
| Sensor Calibration | On-site sensor lab with NIST-traceable standards; force, temperature, and vibration sensors calibrated bi-weekly | ISO/IEC 17025 |
| Final System Validation | 72-hour continuous milling cycle using zirconia, PMMA, and composite blocks | ISO 13485 Annex B |
3. Sensor Calibration Laboratory
Carejoy Digital operates an on-site sensor calibration lab in Shanghai, accredited to ISO/IEC 17025. The lab ensures:
- Bi-weekly recalibration of load cells, thermal sensors, and spindle vibration monitors.
- Real-time drift correction via AI-powered predictive maintenance algorithms.
- Traceability to national standards through partnerships with CNAS (China National Accreditation Service).
This closed-loop calibration ecosystem reduces field failure rates by 41% (2025 internal audit data).
4. Durability & Environmental Testing
The up3d p53 undergoes accelerated life testing simulating 5+ years of clinical use:
| Test Type | Parameters | Pass Criteria |
|---|---|---|
| Thermal Cycling | 5°C to 40°C over 1,000 cycles | No spindle drift > 2µm |
| Vibration Endurance | 10–500 Hz, 500 hours | No mechanical play in guideways |
| Milling Cycle Stress Test | 10,000+ cycles with full zirconia blocks | Surface finish Ra < 0.2µm maintained |
| Dust & Debris Resistance | IEC 60529 IP5X simulation | No ingress into motor housing |
5. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the global leader in the cost-performance optimization of digital dental hardware due to:
- Integrated Supply Chains: Concentrated ecosystem of precision component manufacturers (e.g., ball screws, encoders, motors) within 100km of Shanghai, reducing logistics and inventory costs by up to 30%.
- Advanced Automation: High adoption of Industry 4.0 principles—machine learning-driven predictive maintenance, IoT-enabled production monitoring—increases yield and reduces scrap.
- Skilled Engineering Talent Pool: Over 600,000 annual STEM graduates enable rapid R&D iteration. Carejoy Digital’s R&D team includes specialists from Tongji University and Shanghai Jiao Tong.
- Regulatory Efficiency: NMPA (National Medical Products Administration) streamlines domestic certification while maintaining alignment with EU MDR and FDA Class II equivalency pathways.
- Economies of Scale: High-volume production lowers per-unit cost without sacrificing precision—up3d p53 delivers sub-5µm accuracy at 40% below comparable German or Swiss systems.
Conclusion
The up3d p53 from Carejoy Digital represents the new benchmark in high-precision, cost-effective digital dental milling. Backed by ISO 13485 certification, rigorous sensor calibration protocols, and extensive durability validation, it delivers clinical reliability with unmatched ROI. As China continues to dominate the intersection of precision manufacturing and intelligent design, dental labs and clinics gain access to enterprise-grade technology at democratized pricing.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Up3D P53 Milling Machine.
✅ Open Architecture
Or WhatsApp: +86 15951276160
