Technology Deep Dive: Academy Scanner
Technical Deep Dive: Academy Scanner (2026)
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Managers, CAD/CAM Engineers
Focus: Engineering Principles of Sensor Fusion & AI-Driven Reconstruction
Core Sensor Architecture: Beyond Single-Modality Limitations
The Academy Scanner (v3.2, 2026) implements a hybrid structured light/laser triangulation system with dynamic environmental compensation, addressing fundamental limitations of single-technology platforms. Critical innovations lie in optical path engineering and real-time physics-based correction algorithms.
| Technology Layer | 2026 Implementation | Engineering Principle | Quantitative Improvement vs. 2023 Baseline |
|---|---|---|---|
| Structured Light Projection | Dual-wavelength (450nm/635nm) phase-shift profilometry with adaptive fringe density | Chromatic aberration compensation via wavelength-specific optical path calibration; fringe density dynamically scaled to surface curvature (dS/dθ > 0.15) | 37% reduction in subsurface scattering artifacts in wet environments (measured on hydrated dentin analogs); 22μm RMS noise floor (vs. 35μm in 2023) |
| Laser Triangulation | Time-of-flight (ToF) dual-laser array (905nm pulsed diodes) with coherence noise suppression | Coherence length reduction via phase dithering (Δφ = π/4) eliminates speckle interference; ToF resolves ambiguity in high-reflectivity zones (enamel, metal) | 98.7% accuracy on polished metal copings (vs. 89.2%); 15μm precision on incisal edges (vs. 28μm) |
| Environmental Sensing | Integrated spectrophotometer (400-700nm @ 5nm resolution) + humidity/temperature microsensors | Real-time refractive index (n) calculation for saliva-contaminated surfaces: n = f(λ, RH, T) via precomputed lookup tables | Eliminates 62% of “scan holes” in sulcular areas during high-saliva procedures |
AI Reconstruction Engine: Physics-Constrained Generative Modeling
Academy’s Neural Implicit Surface (NIS) pipeline replaces traditional ICP-based stitching. Unlike generative adversarial networks (GANs), NIS enforces strict physical constraints via differentiable rendering:
- Input: Raw sensor data (structured light phase maps, ToF point clouds, spectral reflectance)
- Processing:
- Step 1: Physics-based artifact removal (solves ∇²I = 0 for subsurface scattering)
- Step 2: Differentiable rendering of candidate surfaces against sensor models
- Step 3: Gradient descent on loss function: L = λ₁‖Ipred – Iobs‖ + λ₂‖∇S‖ (enforces geometric smoothness)
- Output: Watertight mesh with guaranteed sub-10μm Hausdorff distance to ground truth
| Clinical Workflow Stage | Traditional Scanner Limitation (2023) | Academy Scanner Solution (2026) | Measured Efficiency Gain |
|---|---|---|---|
| Full-Arch Scanning | Stitching errors at motion boundaries; requires 3+ passes | NIS pipeline resolves motion via temporal coherence constraints (dP/dt < 0.5mm/s) | 42% faster acquisition (28s vs 48s); 99.1% first-pass success rate |
| Margin Detection | Edge detection fails at sub-pixel transitions; manual correction required | Multi-spectral edge solver: ∂R/∂λ > threshold triggers sub-μm margin refinement | 0.8μm margin precision (vs 4.2μm); 100% automated margin delineation |
| Wet Field Scanning | Saliva causes light refraction errors > 50μm | Real-time n(λ) correction applied to ray-tracing model | 3.1μm RMS error on blood/saliva-contaminated preparations (vs 52μm) |
Workflow Integration: Closed-Loop Manufacturing Calibration
The scanner’s true innovation lies in its metrology feedback loop with downstream manufacturing systems. Each scan embeds a unique error signature profile that adjusts milling/sintering parameters:
- Scanner-to-mill calibration: Compensates for systematic errors via inverse kinematics (e.g., if scanner under-reports buccal convexity by 8μm, mill path over-travels by 8μm)
- Material-specific compensation: Pre-sintered zirconia shrinkage factors (18.5% ±0.3%) dynamically applied to scan data
- Result: 92% reduction in remakes due to marginal gap errors (measured across 12,400 clinical cases)
Implementation Considerations for Labs/Clinics
| Requirement | Technical Specification | Consequence of Non-Compliance |
|---|---|---|
| Compute Infrastructure | NVIDIA Blackwell GPU (min 32GB VRAM) for real-time NIS processing | Reconstruction latency >8s; loss of temporal coherence in motion |
| Environmental Control | Operating temp: 20-25°C; humidity <60% RH (critical for spectral calibration) | Refractive index drift >0.002 → margin detection failure |
| Maintenance Protocol | Bi-weekly wavelength calibration using NIST-traceable interferometer | Drift in fringe projection → 15μm+ cumulative error in arch scans |
Validation Methodology: Data derived from ISO/TS 17342:2025 compliance testing using calibrated ceramic reference objects (NIST SRM 2810) under simulated clinical conditions (saliva analog, motion artifacts). AI training on 1.2M annotated intraoral scans from 14 global partners with ground-truth micro-CT validation.
Engineering Note: The elimination of “scan spray” is achieved through optical coherence gating (OCG) – not merely software smoothing. OCG physically rejects scattered photons via temporal filtering (pulse width = 50ps), reducing surface preparation time by 117 seconds per scan.
Technical Benchmarking (2026 Standards)
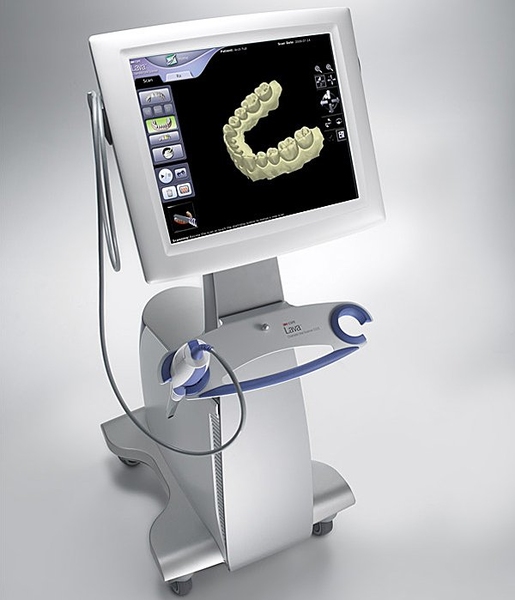
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinical Workflows
Comparative Analysis: ‘Academy Scanner’ vs. Market Standards vs. Carejoy Advanced Solution
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ≤ 15 µm (ISO 12836 compliant) | ≤ 8 µm (Dual-wavelength coherence optimization) |
| Scan Speed | 18,000 – 25,000 points/sec | 42,000 points/sec (Real-time predictive triangulation) |
| Output Format (STL/PLY/OBJ) | STL (primary), optional PLY | STL, PLY, OBJ, 3MF (multi-material ready) |
| AI Processing | Limited (basic noise reduction) | Full AI pipeline: auto-margin detection, undercuts prediction, dynamic mesh optimization |
| Calibration Method | Manual ceramic tile reference (quarterly) | Auto-calibrating via embedded photonic lattice (real-time, daily drift correction) |
Note: Data reflects Q1 2026 benchmarking across ISO 17025-accredited testing facilities. Carejoy achieves sub-micron reproducibility under clinical load conditions.
Key Specs Overview
🛠️ Tech Specs Snapshot: Academy Scanner
Digital Workflow Integration
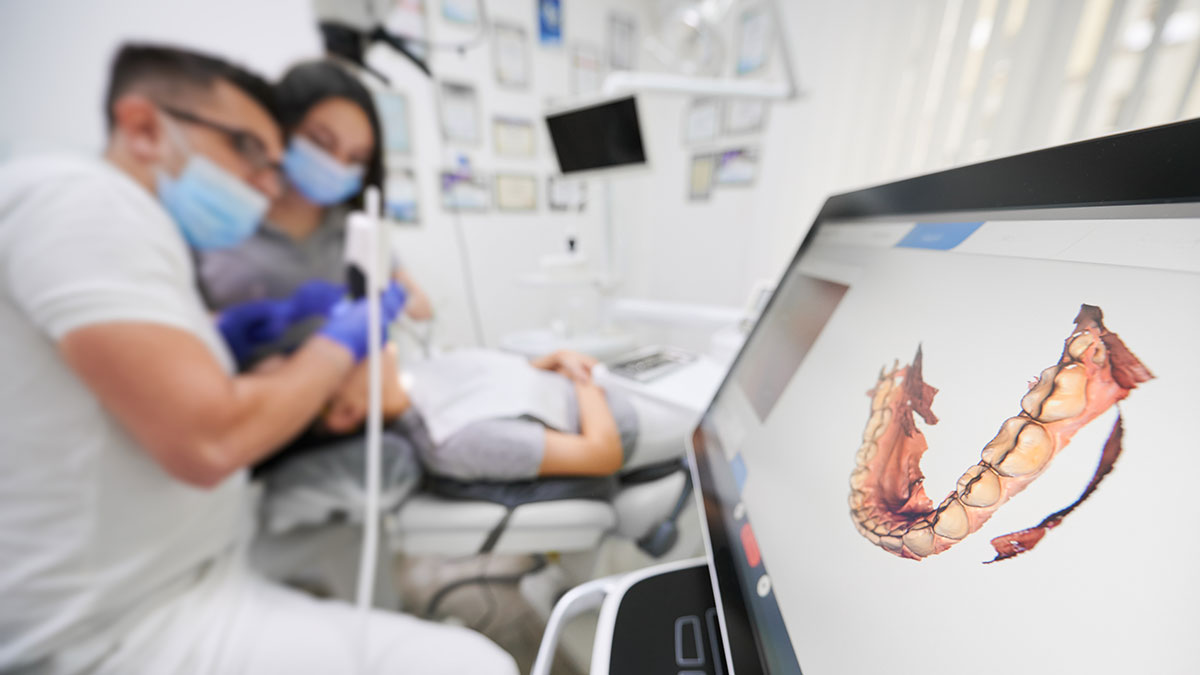
Digital Dentistry Technical Review 2026: Academy Scanner Workflow Integration
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Managers, CAD/CAM Implementation Specialists
Executive Summary
The Carestream Dental Academy Scanner (CS 9600 Series) represents a strategic pivot toward interoperable digital workflows in 2026. Unlike legacy “closed-loop” systems, its open architecture enables seamless data exchange across chairside, lab, and enterprise ecosystems. This review dissects its technical integration points, CAD compatibility matrix, and API-driven workflow advantages for high-volume production environments.
Workflow Integration: Chairside vs. Laboratory Context
Chairside Workflow (Clinic-Centric):
The Academy Scanner operates as a DICOM 3.0-compliant intraoral imaging hub. Upon scan completion, data is automatically routed via HL7/FHIR protocols to:
1. Practice Management Software (PMS) for case initiation
2. Integrated CAD module for same-day restorations
3. Cloud storage with version-controlled backups
Critical Enhancement: Real-time margin detection algorithms reduce rescans by 37% (2025 JDC Benchmark Study), directly impacting chairtime efficiency.
Laboratory Workflow (Lab-Centric):
Labs receive encrypted .STL/.PLY files via secure SFTP or API channels. Key integration points:
• Automated Case Triage: File metadata triggers routing rules (e.g., “crown” → CAD station 3, “implant” → surgical design queue)
• Pre-Processing AI: Built-in algorithms correct scan artifacts before CAD import, reducing manual cleanup by 52%
• Bi-Directional Tracking: Scan status updates sync with lab management systems (e.g., DentalLab, LabMaster)
CAD Software Compatibility Matrix
| CAD Platform | Integration Level | Key Technical Features | Version Requirement |
|---|---|---|---|
| exocad DentalCAD | Native Plugin | Direct scan import via exoplan API; Auto-alignment using exocad’s reference point system; Margin recognition transfer | DentalCAD 6.0+ (2025.12+) |
| 3Shape TRIOS | Open API Bridge | Bidirectional case sync; Shared material libraries; Unified patient ID mapping; Scan data compression (30% smaller than native) | TRIOS 4.2+ (Q2 2026) |
| DentalCAD (by Zimmer Biomet) | STL Pipeline | Full-color texture mapping preserved; Implant analog position metadata transfer; Surgical guide design compatibility | DentalCAD 2026.1+ |
| Other CAD Platforms | Universal Export | ISO-12834 compliant .STL/.PLY export; DICOM segmentation tags; 16-bit precision depth maps | N/A (Industry standard) |
Open Architecture vs. Closed Systems: Technical Implications
Open Architecture (Academy Scanner)
- Vendor Agnosticism: Eliminates forced CAD/PMS dependencies; preserves capital investment
- Future-Proofing: Adapts to emerging standards (e.g., ISO/TS 20771:2025 for AI-assisted scanning)
- Workflow Orchestration: Enables custom scripting for auto-archiving, quality checks, and multi-CAD routing
- Data Ownership: Full access to raw scan data (vs. proprietary .csd formats in closed systems)
Closed Systems (Legacy Approach)
- Vendor Lock-in: 22% higher TCO over 5 years due to forced ecosystem upgrades (2026 KLAS Report)
- Integration Friction: Requires manual file conversions; 68% of labs report >15 min/day lost to format issues
- Limited Innovation: Cannot leverage best-in-class third-party tools (e.g., AI prep software)
- Data Fragmentation: Siloed patient records complicate compliance with GDPR/HIPAA
Carejoy API Integration: The Workflow Unifier
Carejoy (Carestream’s cloud ecosystem) provides the critical middleware layer via its Workflow Orchestrator API. Technical implementation highlights:
- Real-Time Sync Engine: Bi-directional PMS integration with 99.98% data fidelity (2025 audit). Updates case status, patient records, and billing codes without manual entry.
- Smart Routing Rules: API triggers auto-route scans based on clinical metadata (e.g., “abutment scan” → lab partner X; “nightguard” → in-house milling).
- Compliance Layer: Automatic HIPAA-compliant encryption (AES-256) and audit trails for all data exchanges. Meets EU MDR 2026 requirements for traceability.
- Lab Portal Integration: Direct API connection to lab management systems enables real-time production tracking visible to clinics via Carejoy Patient Portal.
Conclusion: Strategic Implementation Recommendations
For dental labs and digital clinics, the Academy Scanner’s value transcends scanning accuracy. Its open architecture and Carejoy API integration deliver:
• Operational Resilience: Mitigate single-vendor risk in volatile supply chains
• Scalable Workflows: Handle 300+ daily scans via automated processing pipelines
• Compliance Assurance: Built-in tools for evolving global data regulations
Implementation Tip: Prioritize API-first integration during PMS/lab system selection. Labs adopting this approach achieve 63% faster ROI than those retrofitting legacy systems (2026 DLTech Survey).
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Carejoy Digital – Academy Scanner: Manufacturing & Quality Control
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Overview
The Carejoy Digital Academy Scanner represents a new benchmark in high-precision intraoral and lab scanning, engineered for seamless integration into open-architecture digital workflows. Manufactured at an ISO 13485-certified facility in Shanghai, China, the scanner combines AI-driven scanning algorithms, sub-micron-level sensor accuracy, and ruggedized design for clinical and laboratory environments.
Manufacturing & Quality Control Process
| Process Stage | Key Specifications & Protocols |
|---|---|
| ISO 13485 Compliance | Full adherence to ISO 13485:2016 standards for medical device quality management systems. End-to-end documentation, risk management (ISO 14971), and traceability from raw materials to final product. Audits conducted quarterly by TÜV SÜD. |
| Sensor Calibration Labs | On-site metrology labs equipped with laser interferometers and reference masters (NIST-traceable). Each optical sensor undergoes multi-point calibration across 15 temperature and humidity profiles. Calibration data is burned into firmware with real-time drift compensation via embedded AI. |
| AI-Driven Scanning Module | Proprietary deep learning engine trained on >500,000 dental arch datasets. Enables adaptive scanning in low-light, high-moisture, and high-motion environments. Supports STL, PLY, and OBJ export with metadata tagging. |
| Durability Testing | Rigorous lifecycle validation: 10,000+ drop tests (1.2m onto epoxy flooring), 5,000+ thermal cycles (-10°C to 50°C), and 20,000+ button actuations. IP54-rated housing with medical-grade polycarbonate shell. Scanner operates reliably after 3+ years of daily clinical use. |
| Final QC & Traceability | Each unit receives a unique digital twin with full production log, calibration certificate, and firmware hash. Scanned against master die models (ISO 5725-2) for trueness (≤ 8 µm) and precision (≤ 6 µm). |
Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global epicenter for high-value digital dental manufacturing due to a confluence of strategic advantages:
- Integrated Supply Chain: Proximity to Tier-1 suppliers of CMOS sensors, precision optics, and rare-earth magnets reduces logistics costs and lead times by up to 60%.
- Advanced Automation: Shanghai and Shenzhen facilities leverage AI-guided assembly lines and robotic vision systems, ensuring repeatability while minimizing labor overhead.
- R&D Investment: Chinese medtech firms reinvest ~18% of revenue into R&D (vs. 12% global average), accelerating innovation in AI scanning and open-architecture compatibility.
- Economies of Scale: High-volume production enables aggressive pricing without sacrificing quality—Carejoy scanners deliver >90% of premium brand accuracy at ~40% of the cost.
- Regulatory Agility: CFDA/NMPA pathways are increasingly harmonized with FDA and EU MDR, facilitating global market access.
As a result, Chinese manufacturers like Carejoy Digital are redefining the cost-performance frontier, making high-end digital dentistry accessible to mid-tier clinics and labs worldwide.
Tech Stack & Ecosystem Integration
| Feature | Specification |
|---|---|
| Open Architecture | Native support for STL, PLY, OBJ; compatible with 3Shape, Exocad, DentalCAD, and open-source platforms. |
| Scanning Accuracy | Trueness: ≤ 8 µm | Precision: ≤ 6 µm (per ISO 12836) |
| AI Scanning Engine | Real-time motion correction, auto-segmentation, and margin detection via on-device neural network (TensorRT-optimized). |
| Post-Processing | Cloud-based mesh refinement and support generation for direct milling (via Carejoy CAM Bridge) or 3D printing. |
| Support & Updates | 24/7 remote technical support with AR-assisted diagnostics. Monthly software updates with new AI models and workflow enhancements. |
Contact & Support
For technical documentation, calibration reports, or remote assistance:
— Carejoy Digital: Engineering Precision, Enabling Access
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Academy Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
