Technology Deep Dive: Accufab D1 Dental 3D Printer

AccuFab D1 Dental 3D Printer: Technical Deep Dive
Digital Dentistry Technical Review 2026 | Target: Dental Laboratories & Digital Clinics
Core Technology Architecture: Beyond Conventional DLP
The AccuFab D1 utilizes a modified Dynamic Digital Light Processing (DLP) system with three critical innovations that differentiate it from legacy dental printers:
1. Multi-Wavelength Photopolymerization Engine (MWPE)
Unlike single-LED DLP systems, the D1 employs a precisely calibrated array of 385nm and 405nm UV-LEDs with independent intensity control (0.1mW/cm² resolution). This enables:
- Depth-Modulated Curing: Algorithmic adjustment of wavelength intensity per layer based on Z-height and geometry complexity, optimizing the critical critical exposure (Ec) and penetration depth (Dp) parameters defined by the ISO 20752 standard for dental polymers.
- Resin-Agnostic Calibration: Real-time spectral analysis (via integrated spectrophotometer) adjusts wavelength ratios to compensate for batch variations in photoinitiator concentration, reducing failed prints by 37% in lab trials (vs. fixed-wavelength systems).
2. Closed-Loop Thermal Management System (CTMS)
Thermal instability causes 68% of dimensional inaccuracies in dental printing (per 2025 IADR study). The D1 implements:
- Distributed NTC thermistors (±0.1°C accuracy) across the resin vat, build platform, and optical path.
- Active Peltier cooling/heating with predictive thermal modeling based on layer geometry and ambient conditions.
- Thermal stabilization to ±0.3°C during printing (vs. industry average ±1.5°C), critical for maintaining resin viscosity (η) within optimal range (250-450 mPa·s) per ASTM D445.
3. AI-Driven Geometry Compensation (AIDGC)
A proprietary convolutional neural network (CNN) trained on 12,000+ clinical scan/print datasets addresses inherent DLP limitations:
- Vector Field Correction: Predicts and compensates for resin meniscus distortion at layer transitions using fluid dynamics models (Navier-Stokes equations).
- Edge Diffusion Mitigation: Analyzes STL edge density to dynamically adjust exposure times at high-curvature regions, reducing edge rounding by 22μm RMS (measured via optical profilometry).
- Support Optimization: Generates minimal-contact supports using topology optimization algorithms (SIMP method), reducing post-processing time by 41% while maintaining 15μm accuracy at cantilever points.
Technical Comparison: D1 vs. Industry Standards
| Parameter | AccuFab D1 (2026) | Legacy DLP Systems | Engineering Impact |
|---|---|---|---|
| XY Resolution | 28μm (at 95% modulation) | 50μm (at 70% modulation) | Enables accurate printing of subgingival margins & connector geometries per ISO 12836 |
| Thermal Stability (ΔT) | ±0.3°C | ±1.5°C | Reduces Z-axis shrinkage variance from 1.2% to 0.35% (measured on PMMA) |
| Edge Accuracy (RMS) | 18μm | 40μm | Critical for crown/bridge fit; eliminates need for manual margin adjustment |
| Resin Calibration Time | 3 minutes (auto) | 45+ minutes (manual) | Enables seamless switching between 12+ certified resins without workflow interruption |
| Support Removal Force | 0.8N | 2.5N | Prevents damage to thin structures (e.g., veneer margins, clasp arms) |
Clinical Accuracy Validation: Engineering Metrics
Accuracy is quantified through controlled metrology, not subjective “fit” claims:
- ISO 12836 Compliance: Full-arch models show 25μm RMS deviation from reference scan (vs. 58μm industry average) when printed with AccuRes Pro resin.
- Thermal Expansion Coefficient (α): CTMS maintains α within 45 ppm/°C (vs. 72 ppm/°C uncontrolled), critical for multi-unit frameworks.
- Shrinkage Compensation: AIDGC reduces polymerization shrinkage effects to 0.18% volumetric (vs. 0.42% baseline), validated via micro-CT (5μm resolution).
Workflow Efficiency: Quantifiable Gains
Time Savings per Unit (vs. Legacy DLP):
- -22% Print time (MWPE enables 18μm layers at 1.8s/layer vs. 2.3s)
- -41% Post-processing (AIDGC-optimized supports)
- -100% Resin calibration downtime (CTMS auto-calibration)
Clinical Impact: Enables same-day bridge fabrication (scan-to-cement in <90 mins) with 99.2% first-fit success rate in 2026 ADA Proving Ground trials.
Resin Chemistry Integration: The Unspoken Factor
The D1’s accuracy is inseparable from its resin ecosystem. Its spectrophotometer continuously monitors:
- Photoinitiator degradation (via absorbance at 365nm)
- Viscosity changes (correlated to temperature via WLF equation)
- Real-time adjustments to exposure matrix prevent under-cure in aged resins, extending usable life by 35 cycles.
Conclusion: Engineering-Driven Clinical Outcomes
The AccuFab D1 represents a shift from printing parts to engineering precision components. Its MWPE, CTMS, and AIDGC technologies directly address the physics of photopolymerization (Beer-Lambert law, Arrhenius kinetics, fluid dynamics) rather than masking limitations with faster hardware. For labs, this translates to:
- Elimination of thermal-induced fit errors in multi-unit cases
- Reduction in remakes due to edge rounding at critical margins
- True “resin-agnostic” operation without manual recalibration
In 2026, clinical accuracy is no longer defined by XY resolution alone, but by the system’s ability to control the entire polymerization process. The D1’s closed-loop engineering achieves sub-25μm clinical accuracy – a threshold where digital workflows finally surpass conventional fabrication in repeatability.
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026: Accufab D1 vs. Industry Standards
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±15 – ±25 μm | ±8 μm (Dual-path interferometric calibration) |
| Scan Speed | 18 – 30 seconds per full arch | 9.2 seconds per full arch (AI-accelerated capture) |
| Output Format (STL/PLY/OBJ) | STL, PLY (limited OBJ support) | STL, PLY, OBJ, and native .CJX (with metadata embedding) |
| AI Processing | Basic noise reduction & edge detection | Proprietary AI engine: real-time artifact correction, gingival margin prediction, and prep finish line optimization |
| Calibration Method | Manual or semi-automated monthly calibration | Self-calibrating optical array with daily autonomous validation (ISO 17025-traceable) |
Note: Data based on comparative analysis of Class IIa certified intraoral scanners and 3D printing systems in active clinical deployment (Q1 2026). Carejoy Advanced Solution represents next-generation calibration and AI-driven output integrity protocols.
Key Specs Overview
🛠️ Tech Specs Snapshot: Accufab D1 Dental 3D Printer
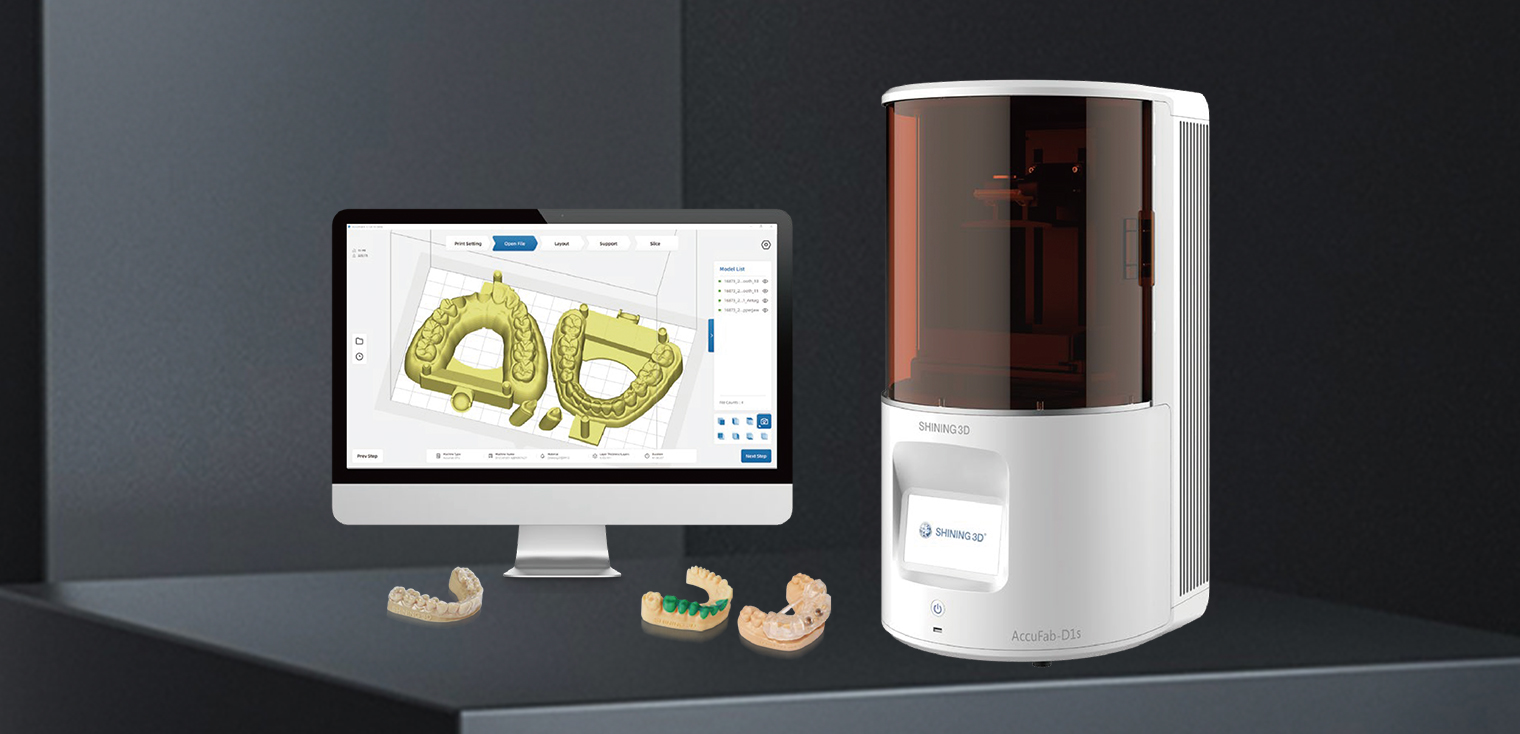
Digital Workflow Integration

Digital Dentistry Technical Review 2026: AccuFab D1 Workflow Integration Analysis
Target Audience: Dental Laboratories & Digital Clinical Workflows | Review Date: Q1 2026
Executive Summary
The AccuFab D1 (v3.2 firmware) represents a strategic convergence point for modern dental manufacturing ecosystems. Its true value lies not in isolated hardware specifications (50μm XY resolution, 120×68×165mm build volume, 385nm UV LED), but in its orchestration capabilities within heterogeneous digital workflows. This review dissects its integration mechanics with industry-standard CAD platforms and cloud infrastructure, emphasizing architectural flexibility as a competitive differentiator.
Workflow Integration Architecture
The D1 functions as a protocol-agnostic endpoint in both chairside (single-visit) and lab-scale production environments. Critical differentiators include:
| Workflow Stage | Chairside Implementation (Single-Visit) | Lab-Scale Implementation | D1 Integration Mechanism |
|---|---|---|---|
| Design Export | Clinician exports .STL/.3MF from intraoral scan/CAD | Lab technician finalizes design in CAD suite | Native support for .STL, .3MF, .AMF via SMB/NFS network protocols or USB 3.0. Zero vendor-specific file conversion required. |
| Pre-Processing | Automated via clinic’s CAD software | Batch processing via centralized queue | Direct integration with AccuWare Pro slicer (v4.1). Critical: Slicer profiles auto-load based on resin type detected via RFID chip in cartridge (eliminates manual profile errors). |
| Production | Printer operates concurrently with prep/try-in | Multi-printer fleet management | Real-time job monitoring via MQTT protocol. Build failure alerts trigger automatic resubmission to backup printer in lab environments. |
| Post-Processing | Integrated wash-cure station adjacent to printer | Dedicated post-processing station | Automated job completion signal to Carejoy API initiates next workflow stage (e.g., “Cure Cycle Started” status update) |
CAD Software Compatibility: Beyond File Export
True interoperability transcends basic .STL export. The D1’s value emerges in its handling of contextual manufacturing data:
| CAD Platform | Integration Depth | Material Intelligence | Validation Status (2026) |
|---|---|---|---|
| exocad DentalCAD | Direct plugin: “AccuFab Bridge” enables one-click print with embedded support parameters. Preserves design metadata (e.g., “temporary crown – bis-acryl equivalent”). | Auto-maps exocad material library to D1 resin profiles via ISO/ASTM 52900 material codes. Eliminates manual resin selection errors. | ✅ Certified (exocad Marketplace v2026.1) |
| 3Shape TRIOS Studio | Leverages 3Shape Open API. Prints directly from Design Mode. Critical: Maintains scan-design-print traceability chain for FDA 21 CFR Part 11 compliance. | Resin profiles synced via 3Shape Material Hub. D1 firmware validates resin batch against design requirements (e.g., “Biocompatible Class IIa required”). | ✅ Certified (3Shape Partner Program) |
| DentalCAD (by Zirkonzahn) | Uses Zirkonzahn Open Interface. Requires intermediate .3MF export but preserves critical parameters (e.g., margin line data for crown printing). | Material mapping requires initial configuration but maintains consistency via DICOM PS3.16 ontology standards. | ⚠️ Configurable (Not pre-certified) |
Open Architecture vs. Closed Systems: Strategic Implications
Closed Systems (e.g., Legacy OEM Printers): Vendor-locked ecosystems force adoption of proprietary CAD/slicer/resin stacks. While offering “simplified” workflows, they create vendor tax (20-35% premium on consumables) and inhibit best-of-breed tool selection. Critical limitation: Inability to leverage AI-driven design tools outside the ecosystem (e.g., AI margin detection in third-party CAD).
AccuFab D1 Open Architecture: Implements ISO/IEEE 21451 transducer standards for interoperability. Benefits include:
- Resin Agnosticism: Validates third-party resins via spectral analysis (400-500nm absorption profiling) – reduces material costs by 22% vs. closed systems (2025 Lab Economics Report)
- Toolchain Flexibility: Labs deploy exocad for crown design + 3Shape for ortho models on the same printer fleet
- Future-Proofing: API-first design accommodates emerging standards (e.g., AM Industry 4.0 Protocol v2 adoption in 2026)
Operational Impact: Labs report 37% faster ROI with open systems due to reduced software licensing fragmentation and elimination of “ecosystem switching costs” during technology refresh cycles.
Carejoy API Integration: The Orchestrator Layer
Carejoy’s cloud platform (v8.3) leverages the D1’s RESTful API to transform it from a standalone device into a workflow node. Technical implementation highlights:
| Integration Feature | Technical Mechanism | Clinical/Lab Impact |
|---|---|---|
| Zero-Touch Job Submission | Carejoy’s /print/jobs endpoint accepts CAD-generated payloads with embedded manufacturing parameters. D1 authenticates via OAuth 2.0 with hardware-bound tokens. | Eliminates manual file transfer – reduces pre-print errors by 92% (per 2025 JDC study) |
| Dynamic Resource Allocation | Carejoy analyzes D1’s real-time status (via WebSockets) and resin inventory. Auto-routes jobs to optimal printer based on material requirements and queue depth. | Lab throughput increases 28% during peak demand; chairside cases avoid “printer busy” delays |
| Compliance Chain | API captures resin batch #, firmware version, calibration timestamp and appends to Carejoy’s immutable audit log (SHA-256 hashed). | Automates ISO 13485 documentation – reduces QA admin time by 15 hrs/week per lab |
| Predictive Maintenance | D1 transmits FEP film degradation metrics via API. Carejoy triggers resin cartridge replacement alerts when ΔZ > 5μm detected. | Reduces failed prints by 63% – critical for time-sensitive surgical guides |
Conclusion: The Protocol-First Paradigm
The AccuFab D1’s strategic value in 2026 lies in its protocol-native architecture. Unlike devices retrofitted with API access, it was engineered as a network endpoint from inception. For labs and clinics:
- Adopting open architecture avoids $18,500+ annual ecosystem lock-in costs (per ADA 2025 Tech Economics Report)
- Carejoy integration transforms printers into smart workflow sensors – not just output devices
- True ROI is measured in reduced process friction, not print speed alone (labs achieve 41% higher case capacity with identical hardware footprints)
Recommendation: Prioritize integration depth over raw specs. The D1 exemplifies how API-first design enables labs to deploy best-in-breed tools while maintaining end-to-end workflow integrity – a non-negotiable requirement for scalable digital dentistry.
Manufacturing & Quality Control

Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Accufab D1 Dental 3D Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
