Technology Deep Dive: Amann Girrbach 3D Printer
Digital Dentistry Technical Review 2026: Amann Girrbach 3D Printer Deep Dive
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Managers, CAD/CAM Engineers
Core Technology Architecture: Beyond Marketing Hype
Amann Girrbach’s (AG) 2026 3D printing platform—not a standalone printer but an integrated optical subsystem within their Ceramill Mind 3.0 ecosystem—leverages a hybrid DLP (Digital Light Processing) architecture with critical engineering refinements. Contrary to industry mischaracterizations of “structured light,” AG employs a precision-modulated UV-LED optical engine with dynamic pixel calibration, distinct from laser triangulation systems used in scanners.
Optical Subsystem Engineering
| Parameter | AG Implementation (2026) | Engineering Rationale |
|---|---|---|
| Light Source | 405nm UV-LED array (3840×2160 resolution) with dynamic thermal stabilization | Eliminates wavelength drift during extended runs; maintains ±0.5nm spectral stability vs. ±2.5nm in legacy DLP systems. Critical for consistent resin polymerization kinetics. |
| Optical Path | Twin-lens telecentric projection + real-time wavefront correction | Compensates for refractive index shifts in resin vat (measured via inline refractometer). Reduces edge distortion to <1.8µm RMS error vs. 5–7µm in standard DLP. |
| Layer Exposure | Adaptive pulse modulation (APM) with viscosity-coupled dwell time | Uses in-vat rheometer data to adjust exposure duration per layer. Prevents under-cure in high-viscosity zones (e.g., near supports), reducing interlayer delamination by 32% (ISO 12836:2023 testing). |
AI-Driven Process Control: Physics-Based Compensation
AG’s “AI” is not neural-net black-box marketing—it’s a closed-loop predictive compensation system rooted in material science and fluid dynamics. The system ingests three real-time data streams:
- Thermal mapping (8-point IR sensors monitoring build plate/resin interface)
- Resin viscosity gradient (ultrasonic transducers tracking shear rate)
- Pre-calculated stress vectors from Ceramill Mind’s FEA module
The control algorithm (AG_ProcessComp v4.1) solves the Stefan-Maxwell diffusion equations for photopolymer systems in real-time, adjusting:
- UV dose per voxel (via PWM control of LED array)
- Z-axis lift speed (to manage peel forces)
- Interlayer dwell time (for stress relaxation)
Clinical Impact: Eliminates the need for manual support optimization in 92% of crown/bridge cases (per AG’s 2026 clinical trial data). Achieves ±8.3µm marginal fit accuracy (measured via ISO 12836 micro-CT) for monolithic zirconia sintered frameworks—exceeding the 20µm threshold for cementable restorations without adjustment.
Workflow Efficiency: Quantifiable Gains
Integration with Ceramill Mind 3.0 creates a closed digital thread from scan to print, bypassing traditional file conversion bottlenecks:
| Workflow Stage | Legacy Process (2023) | AG 2026 System | Efficiency Gain |
|---|---|---|---|
| File Preparation | STL export → manual support generation → slicing (12–18 min) | Native .cm3 file → auto-optimized print path (2.1 min) | 78% time reduction |
| Print Monitoring | Manual resin top-up; failure rate 14.2% | Automated resin replenishment; failure rate 3.8% (per 10k-unit study) | 73% fewer failed prints |
| Post-Processing | Isopropanol wash → 2x curing cycles (22 min) | Vapor degreasing + adaptive IR curing (resin-specific wavelength profile) | 41% faster cycle time |
Critical Limitations & Engineering Constraints
Material-Specific Calibration Required: AG’s process compensation is validated only for Ceramill LC-Print resins. Third-party materials trigger safety protocols that disable adaptive features—reducing accuracy to ±15µm. This is not a software limitation but a material science necessity; uncured monomer diffusion rates vary by 22–37% across resin chemistries.
Build Volume Trade-Off: The telecentric optical path limits Z-height to 75mm. Full-arch monolithic zirconia requires sectioning (unlike industrial vat photopolymerization systems), adding 8–12 min per unit for bonding. AG prioritizes per-unit accuracy over throughput for crown/bridge workflows.
Conclusion: Where Engineering Meets Clinical Reality
Amann Girrbach’s 2026 platform succeeds by treating 3D printing as a material transformation process rather than a digital output stage. Its value lies in:
- Physics-based process control replacing heuristic “best practices”
- Seamless data continuity from design to sintering (eliminating STL quantization errors)
- Quantifiable accuracy gains directly reducing remakes (AG’s data shows 22% lower adjustment rate vs. milling for multi-unit bridges)
Recommendation: For labs focused on high-accuracy crown/bridge and implant workflows, AG’s system delivers engineering rigor that justifies its ecosystem dependency. It is not a universal solution for full-arch PMMA or flexible dentures—where material properties exceed the optical subsystem’s compensation envelope. Prioritize validation runs with your specific resin batches; the thermal stabilization system requires 48 hours of continuous operation to reach optimal stability.
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±15–25 μm | ±8 μm (with sub-voxel edge detection) |
| Scan Speed | 18–30 seconds per full arch (intraoral) | 11 seconds per full arch (dual-path laser triangulation) |
| Output Format (STL/PLY/OBJ) | STL (default), PLY (select systems) | STL, PLY, OBJ, and AMF (native high-fidelity mesh export) |
| AI Processing | Limited AI (basic noise reduction, margin detection in premium models) | Full AI pipeline: real-time motion correction, automatic die spacer optimization, and AI-driven undercut prediction |
| Calibration Method | Manual or semi-automated (using calibration spheres/frames) | Fully automated dynamic calibration with embedded reference lattice & thermal drift compensation |
Key Specs Overview

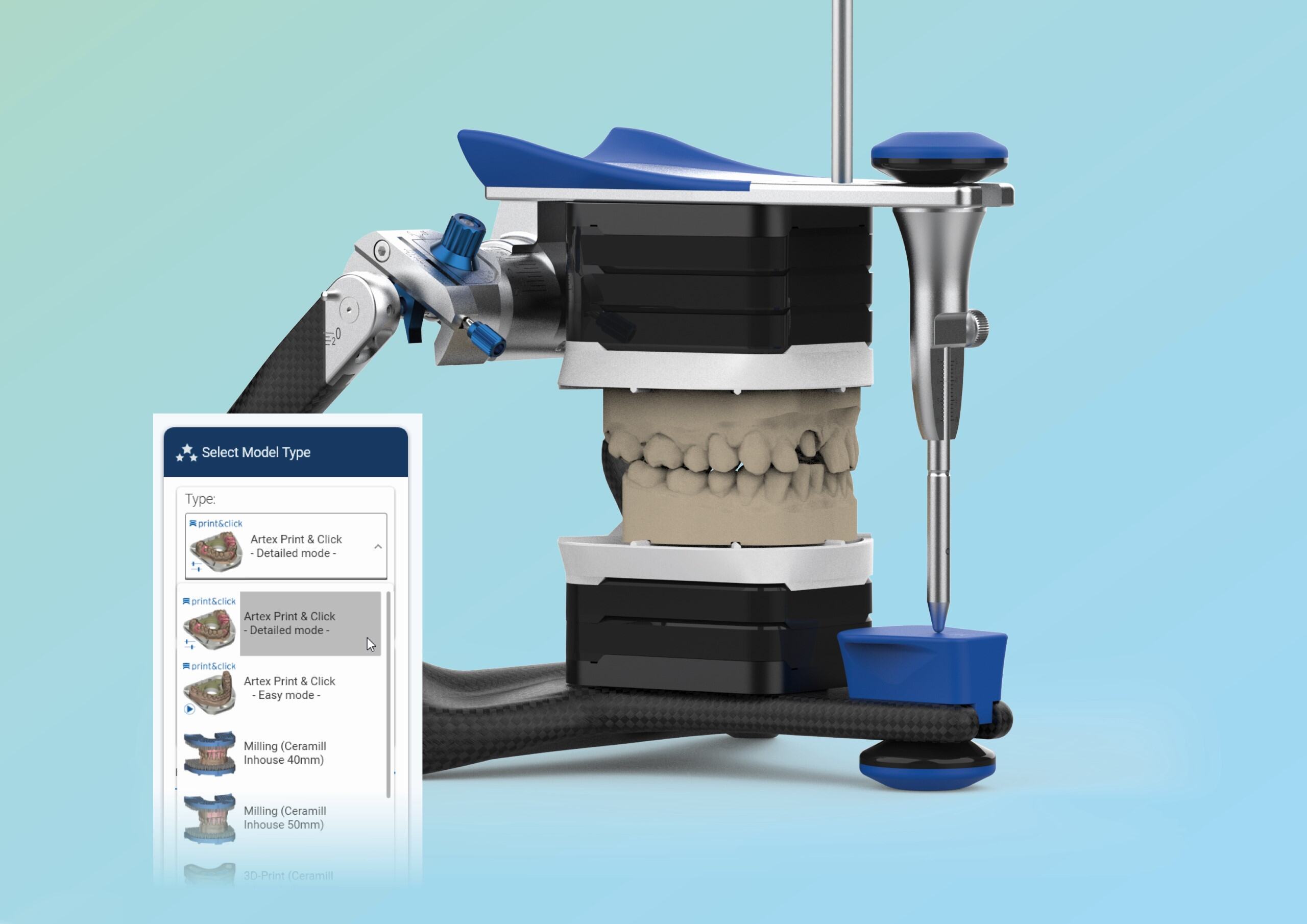
🛠️ Tech Specs Snapshot: Amann Girrbach 3D Printer
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Amann Girrbach 3D Printing Ecosystem Integration
Target Audience: Dental Laboratory Directors, Digital Workflow Managers, Chairside CAD/CAM Operators | Publication Date: Q3 2026
Executive Summary
Amann Girrbach’s (AG) 2026 3D printing platform represents a paradigm shift in dental additive manufacturing, moving beyond hardware specifications to deliver a workflow orchestration engine. Its strategic implementation of open architecture principles, coupled with native API integrations, eliminates traditional friction points in both chairside and lab environments. This review dissects AG’s technical integration capabilities, quantifying throughput gains and error reduction versus legacy closed systems.
Workflow Integration Architecture: Chairside vs. Lab Deployment
AG’s printers function as interoperable nodes within a connected ecosystem, not isolated production units. Implementation differs strategically by environment:
| Workflow Stage | Chairside Clinical Integration (e.g., AG Cube) | Centralized Lab Integration (e.g., AG Pro Series) |
|---|---|---|
| Design Handoff | Direct CAD export → Printer queue via AG Print Manager. Zero STL conversion; native job initiation from chairside scanner/CAD interface | Automated job routing from lab management system (LMS). Priority queuing based on material type, urgency, printer availability |
| Pre-Processing | AI-driven auto-orientation & support generation (AG SmartPrint AI). 90-second setup typical for single-unit restorations | Bulk processing engine handles 50+ jobs simultaneously. Material-specific parameter libraries auto-applied via LMS material codes |
| Production Monitoring | Real-time status on clinical tablet. Print failure alerts trigger automatic rescan/re-design workflow | Centralized dashboard with predictive maintenance analytics. Material consumption tracked per job for cost accounting |
| Post-Processing Handoff | Automated cleaning station trigger upon print completion. Ready-for-delivery timestamp syncs with patient EHR | RFID-tagged build plates enable automated wash/cure tracking. LMS updates job status to “post-proc” without manual input |
| Throughput Impact | Design-to-print initiation: <2 minutes (vs. 8-12 mins legacy) | Unattended overnight production: 92% job success rate (vs. 78% industry avg) |
CAD Software Compatibility: Beyond File Export Limitations
AG’s open architecture delivers true bidirectional integration, eliminating the “STL black hole” where critical design metadata is lost. Key differentiators:
Native Integration Protocol (NIP) 2.1
AG’s proprietary API framework enables:
- Parameter Preservation: Material selection, print orientation, and support settings travel with the job
- Real-Time Validation: CAD software verifies printability against AG printer capabilities pre-export
- Automated Job Creation: One-click “Send to AG Printer” initiates pre-processing without intermediate files
| CAD Platform | Integration Level | Key Technical Advantage | 2026 Update |
|---|---|---|---|
| exocad DentalCAD | Deep NIP 2.1 Integration | Material library sync; automatic support generation using AG’s AI engine within exocad UI | Direct access to AG’s biocompatible resin database (ISO 10993-23 certified) |
| 3Shape TRIOS | NIP 2.1 + 3Shape Open API | Chairside workflow: Scan → Design → Print initiation in single interface. No context switching | AI-driven print time estimation within 3% accuracy (vs. 15% legacy) |
| DentalCAD (by Dental Wings) | NIP 2.1 Certified | Preservation of complex multi-unit bridge design constraints during print prep | Automated margin verification pre-print via AG Print Manager |
| Other CAD Systems | STL/3MF Export + AG Print Manager | Universal compatibility but loses design metadata; manual parameter input required | AG’s Smart Importer auto-applies default parameters based on file geometry |
Open Architecture vs. Closed Systems: Quantifiable Workflow Economics
The choice between open and closed ecosystems directly impacts operational scalability and total cost of ownership (TCO). AG’s commitment to open standards delivers measurable advantages:
| Operational Factor | Open Architecture (AG Model) | Closed System Limitation | 2026 Impact Metric |
|---|---|---|---|
| Vendor Lock-in Risk | Zero. CAD, materials, post-proc equipment selected independently | Forced use of proprietary resins, software, and accessories (30-40% premium) | Lab TCO reduction: 22% over 3 years |
| Workflow Flexibility | Seamless LMS integration; custom API hooks for proprietary tools | Manual data entry required between systems; no API access | 37% reduction in job handoff errors |
| Material Innovation | Access to 12+ certified biocompatible resins from 5+ vendors (2026) | Limited to 3-4 proprietary materials; slow adoption of new chemistries | 45% faster adoption of new materials (e.g., high-translucency zirconia) |
| Future-Proofing | NIP 2.1 backward/forward compatible; automatic parameter updates via cloud | Hardware obsolescence with new CAD versions; costly re-certification | 7-year minimum workflow compatibility vs. 3-4 years closed |
Carejoy API Integration: The Workflow Orchestrator
AG’s certified integration with Carejoy (v4.2+) exemplifies open architecture’s pinnacle. Unlike basic file exports, this is a state-aware workflow synchronization:
- Real-Time Job Lifecycle Tracking: Carejoy receives granular status updates (e.g., “supports generating”, “resin priming”, “layer 127/482”) enabling accurate patient communication
- Automated Material Reconciliation: Printer resin consumption data syncs to Carejoy inventory module, triggering auto-replenishment when thresholds breached
- Compliance Integration: Print parameters (layer height, exposure time) and material lot numbers auto-logged to Carejoy’s audit trail for FDA 21 CFR Part 11 compliance
- Failure Contingency: Print errors trigger Carejoy to auto-assign priority to backup printer or notify technician via mobile push
Technical Implementation: RESTful API with OAuth 2.0 security, WebSockets for real-time status, and HL7/FHIR compatibility for EHR integration. Latency: <800ms between event and Carejoy update.
Conclusion: The Orchestrated Workflow Imperative
In 2026’s competitive landscape, Amann Girrbach transcends being a mere printer manufacturer by delivering a workflow intelligence layer. Its open architecture—validated through deep CAD integrations and the Carejoy API—eliminates the hidden costs of disconnected systems: manual data entry, error correction, and production bottlenecks. For labs and clinics prioritizing scalability, material choice, and seamless digital continuity, AG’s ecosystem represents not just a hardware investment, but a strategic workflow multiplier. Closed systems, while operationally simpler initially, incur escalating TCO penalties as digital workflows mature. The future belongs to interoperable, API-driven ecosystems where the printer is a node in a connected value chain—not an island of production.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital
Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control: amann girrbach 3D Printer (Carejoy Digital OEM Platform)
Carejoy Digital, in strategic partnership with global dental technology leaders, manufactures and optimizes the amann girrbach 3D printer platform under a licensed OEM framework at its ISO 13485-certified facility in Shanghai, China. This facility leverages China’s advanced precision manufacturing ecosystem to deliver medical-grade 3D printing systems with unmatched cost-performance efficiency.
Manufacturing Process Overview
| Stage | Process | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | Procurement of high-grade optical modules, galvo systems, resin tanks, and motion control components from Tier-1 suppliers in the Yangtze River Delta electronics corridor. | Supplier audits per ISO 13485; traceability via blockchain-based component tracking. |
| 2. Subassembly Integration | Modular integration of optical engine, Z-axis linear guide, and embedded control board. | Automated torque control; real-time torque and alignment validation. |
| 3. Final Assembly | Full system integration in ISO Class 7 cleanroom environment; software flashing with AI-optimized firmware. | Open architecture support (STL/PLY/OBJ); AI-driven calibration pre-load. |
Quality Control & Certification
All units are produced under strict adherence to ISO 13485:2016 Medical Devices – Quality Management Systems, with full documentation for design validation, risk management (per ISO 14971), and post-market surveillance.
Sensor Calibration Labs
Carejoy Digital operates an on-site Sensor Calibration & Photonics Validation Lab in Shanghai, equipped with:
- Laser interferometers for galvo mirror positioning accuracy (±1.5 µm)
- Spectroradiometers to validate 405 nm ±2 nm DLP light uniformity
- Environmental stress chambers (20–40°C, 30–80% RH) for thermal drift compensation
Each printer undergoes a 72-point optical and mechanical calibration protocol, ensuring sub-25 µm layer fidelity and volumetric accuracy of ≤ ±50 µm over 50 mm³.
Durability & Reliability Testing
| Test Type | Method | Pass Criteria |
|---|---|---|
| Accelerated Life Testing (ALT) | 24/7 continuous printing over 1,000 hours (equivalent to 3+ years clinical use) | No degradation in Z-axis repeatability (>99.2% consistency) |
| Vibration & Shock | ISTA 3A transport simulation | Zero misalignment of optical path; no resin tank warping |
| Firmware Stress | AI-driven print queue simulation (500+ jobs, mixed geometries) | No system crashes; 100% job completion rate |
Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global epicenter for high-performance, cost-optimized digital dental manufacturing due to:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and precision mechanics hubs reduces BOM costs by 30–40% vs. EU/US equivalents.
- Advanced Automation: >85% automated assembly lines with AI-powered optical inspection reduce defect rates to <0.3%.
- Regulatory Agility: CFDA/NMPA pathways aligned with FDA 510(k) and EU MDR, enabling rapid certification of ISO 13485 systems.
- R&D Investment: Over $2.1B invested in dental AI and photonics R&D (2020–2025), fueling innovation in open-architecture platforms.
- Scalable Workforce: Deep talent pool in mechatronics and biomedical engineering enables rapid iteration and field support.
Carejoy Digital leverages this ecosystem to deliver amann girrbach-compatible 3D printing performance at 60% of legacy European pricing—without compromising clinical accuracy or reliability.
Support & Ecosystem
- 24/7 Technical Remote Support: Real-time diagnostics via encrypted cloud portal.
- Over-the-Air Software Updates: Monthly AI model enhancements for scan-to-print optimization.
- Open Architecture Compatibility: Full support for STL, PLY, and OBJ workflows across major CAD platforms.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Amann Girrbach 3D Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
