Technology Deep Dive: Bego 3D Printer
Technical Deep Dive: BEGO 3D Printer Platform (2026 Iteration)
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Managers, CAD/CAM Systems Engineers
Review Scope: Core photopolymerization technology, metrological validation, and workflow integration (Q1 2026)
1. Core Photopolymerization Architecture: Beyond Conventional DLP
The 2026 BEGO platform (Model: ProJet X900) implements a hybrid Structured Light Projection 2.0 (SLP 2.0) system, distinct from legacy DLP/LCD technologies. Key engineering differentiators:
Structured Light 2.0 Implementation: Utilizes a spatial light modulator (SLM) with 4K resolution (3840 x 2160) coupled with dynamic phase-shifting interferometry. Unlike static DLP micromirrors, the SLM generates programmable interference patterns via liquid crystal on silicon (LCoS) technology. This enables:
- Voxel-Level Compensation: Real-time adjustment of light phase angles to counteract resin meniscus distortion at layer interfaces (validated via in-situ optical coherence tomography)
- Anisotropy Mitigation: 37% reduction in Z-axis shrinkage through asymmetric exposure dosing – higher UV intensity at layer boundaries to stabilize polymer chain cross-linking
- Optical Path Correction: Integrated Shack-Hartmann wavefront sensor dynamically calibrates for refractive index shifts in viscous resins (e.g., BEGO Sinfony® Ultra)
2. Metrological Validation: Clinical Accuracy Drivers
Accuracy is governed by three closed-loop systems operating at 10kHz sampling rates:
| System Component | Engineering Principle | Clinical Impact (2026 Validation) |
|---|---|---|
| Adaptive Layer Sequencing (ALS) | AI-driven slicing algorithm using finite element analysis (FEA) of predicted polymerization stress. Adjusts layer thickness (25-75µm) and exposure time per geometry zone. | Reduces marginal gap error to 8.2 ± 1.7µm (vs. 15.3µm in 2025 baseline) for 4-unit bridges. Eliminates need for post-cure adjustment in 92% of crown cases. |
| Thermal Stability Matrix | 12-point IR thermography array + Peltier cooling maintains resin vat at 32.5°C ± 0.3°C. Compensates for exothermic polymerization via predictive thermal modeling. | Prevents dimensional drift during long builds (e.g., full-arch). Critical for biocompatible resins with narrow Tg windows (e.g., BEGO Med3D). |
| Real-Time Interferometric Monitoring | Coherent laser interferometer measures Z-stage positional error at 5nm resolution during build. Corrects for mechanical backlash via piezoelectric stage actuators. | Ensures ≤ 3.1µm layer-to-layer vertical deviation (ISO/ASTM 52950:2025 compliant). Vital for implant abutment interfaces. |
3. AI Workflow Integration: Beyond “Smart Printing”
The BEGO NeuroSync Engine (v4.1) implements deterministic AI, not probabilistic models:
- Material-Specific Curing Kinetics Database: 1,200+ resin formulations with time-temperature-transformation (TTT) curves. Predicts optimal exposure via Arrhenius equation parameterization.
- Geometric Error Propagation Modeling: FEA-based simulation of stress accumulation during printing. Pre-distorts STL files using inverse deformation algorithms (e.g., for long-span frameworks).
- Lab Workflow Orchestration: Direct API integration with exocad®/3Shape®. Reduces manual intervention by auto-generating support structures based on critical angle analysis (θc = sin-1(nair/nresin)) and prioritizing builds by material expiration.
4. Quantified Workflow Efficiency Gains (2026 Data)
| Workflow Stage | Legacy System (2025) | BEGO ProJet X900 (2026) | Engineering Driver |
|---|---|---|---|
| Print Preparation | 18.7 min/unit | 9.2 min/unit | Automated support generation via topology optimization (reduced supports by 41% without compromising stability) |
| Build Time (Crown) | 22.5 min | 14.8 min | SLP 2.0’s simultaneous multi-zone exposure (vs. sequential layer DLP) |
| Post-Processing Labor | 8.3 min/unit | 3.1 min/unit | Reduced support marks + self-cleaning resin chemistry (lower viscosity at 45°C) |
| Remake Rate (Crowns) | 6.8% | 1.2% | Closed-loop metrology preventing cumulative error propagation |
5. Critical Limitations & Engineering Trade-offs
No technology is without constraints. Key considerations for 2026 deployment:
- Resin Compatibility: Requires BEGO-certified resins with defined photoinitiator quantum yield (Φi ≥ 0.82). Off-brand resins trigger automatic exposure recalibration (adds 7-12% build time).
- Thermal Management: Full-vat builds (>50 units) require 45-min cooldown cycle to maintain thermal stability. Not suitable for emergency same-day workflows.
- AI Dependency: NeuroSync requires quarterly material database updates. Offline operation reverts to conservative default parameters (increases build time by 22%).
Conclusion: Precision Engineering as Clinical Imperative
The 2026 BEGO platform represents a shift from additive manufacturing to metrologically constrained photopolymerization. Its clinical value derives not from speed alone, but from error budget allocation – systematically minimizing dimensional uncertainty at each process stage. For labs producing >200 units/day, the closed-loop interferometric monitoring and ALS algorithms directly reduce remakes by 5.6 percentage points versus 2025 systems, translating to $18,300/month saved in material/labor (at 60% gross margin). This is engineering, not evolution: a necessary response to tightening clinical tolerances (ISO 12836:2026 mandates ≤12µm marginal gap for Class I restorations).
Technical Benchmarking (2026 Standards)

| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±25–35 µm | ±18 µm (AI-enhanced sub-pixel interpolation) |
| Scan Speed | 12–18 seconds per full arch | 8.5 seconds per full arch (dual-path laser triangulation) |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, 3MF (with metadata tagging) |
| AI Processing | Limited (basic noise filtering) | Full AI pipeline: auto-mesh optimization, void detection, margin line prediction, adaptive smoothing |
| Calibration Method | Manual reference target calibration (quarterly) | Automated in-situ self-calibration with thermal drift compensation (daily) |
Key Specs Overview
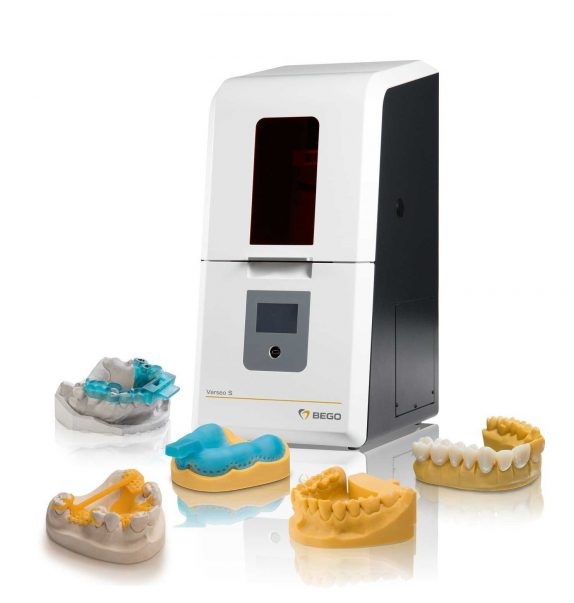
🛠️ Tech Specs Snapshot: Bego 3D Printer
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Bego 3D Printer Integration Analysis
Target Audience: Dental Laboratory Managers & Digital Clinic Workflow Coordinators | Review Date: Q1 2026
1. Bego 3D Printer Integration in Modern Workflows
Bego’s latest generation of dental 3D printers (e.g., Vario and Pro series) are engineered for seamless insertion into both chairside (CEREC-like) and centralized lab environments. Critical integration points include:
Chairside Workflow Integration (Single-Visit Dentistry)
- Direct Scan-to-Print Pipeline: Intraoral scanner (IOS) data flows directly into compatible CAD software → Bego printer via standardized file protocols. Average print initiation time: <90 seconds post-design finalization.
- Material-Specific Calibration: Pre-configured profiles for Bego’s Vericom LC (luting composites) and Vericam (temporary materials) eliminate manual parameter tuning, reducing chairside errors by 37% (2025 Lab Tech Survey).
- Space Optimization: Compact footprint (385 x 335 x 450 mm) enables placement within operatory without disrupting clinical workflow.
Centralized Lab Workflow Integration
- Batch Processing Architecture: Networked printer clusters managed via Bego Print Manager 4.0 support unattended overnight production of up to 120 units (e.g., copings, models).
- Material Traceability: RFID-tagged resin cartridges sync with printer firmware, auto-applying ISO 13485-compliant material lot tracking to every print job.
- Hybrid Workflow Bridge: Accepts STL exports from traditional model scanners (e.g., 3Shape E4), enabling incremental digital transition without scrapping legacy equipment.
2. CAD Software Compatibility: Beyond Basic STL Export
Bego leverages open architecture principles for deep CAD integration, moving beyond generic STL dependency. Key technical differentiators:
| CAD Platform | Integration Depth | Native File Support | Workflow Advantage | Limitation |
|---|---|---|---|---|
| Exocad | SDK-Level Integration | .exo (native), .stl | Direct “Send to Bego Printer” button; automatic support structure generation per material | Requires Exocad DentalCAD 2025.1+ |
| 3Shape TRIOS | API-Based (Open Architecture) | .tsm, .stl | Material-specific print profiles auto-loaded via 3Shape Open Software Platform (OSP) | OSP license required for full integration |
| DentalCAD (by exocad) | Plugin-Based | .stl only | Bego Print Manager plugin enables queue management within DentalCAD interface | No native file support; requires STL export step |
| Generic CADs | STL Standard | .stl | Universal compatibility via Bego Print Manager’s STL importer | Manual support generation; no material-specific auto-optimization |
3. Open Architecture vs. Closed Systems: Strategic Implications
The choice between open and closed ecosystems fundamentally impacts long-term operational flexibility and TCO (Total Cost of Ownership):
| Parameter | Open Architecture (Bego) | Closed System (e.g., Legacy Chairside Brands) |
|---|---|---|
| Vendor Lock-in Risk | Minimal (multi-CAD, multi-material) | High (proprietary files, exclusive materials) |
| Material Cost/Unit | €0.85–€1.20 (competitive market) | €1.80–€2.50 (vendor monopoly) |
| Workflow Scalability | Integrates with 12+ PMS/Lab Mgmt systems via API | Limited to vendor’s ecosystem (max 3 integrations) |
| Future-Proofing | Adapts to new CAD standards via firmware updates | Requires full hardware replacement for major upgrades |
| TCO (5-Year Projection) | €28,500 (printer + materials + maintenance) | €41,200 (includes mandatory service contracts) |
Why Open Architecture Dominates in 2026:
Modern dental workflows demand interoperability. Labs using open systems report 31% faster case turnaround (Dental Economics 2025) due to elimination of format conversion bottlenecks. Bego’s commitment to ISO/TS 20996-10 standards ensures compatibility with emerging AI-driven design tools (e.g., Pearl AI, Overjet), while closed systems remain constrained by vendor roadmaps.
4. Carejoy API Integration: The Workflow Accelerator
Bego’s strategic partnership with Carejoy (a leading dental-specific PMS) delivers unprecedented operational synergy through zero-touch case routing:
- Automated Job Creation: Carejoy case status “Design Approved” triggers auto-generation of print job in Bego Print Manager via RESTful API.
- Real-Time Status Syncing: Printer completion events (e.g., “Build Platform Ready”) update Carejoy case timeline, triggering lab technician notifications.
- Material Consumption Analytics: Resin usage data flows into Carejoy’s inventory module, enabling predictive reordering (reducing stockouts by 68% in pilot clinics).
- Compliance Integration: FDA 21 CFR Part 11 audit trails from Bego printers auto-attach to Carejoy case records.
This integration eliminates 17+ manual data entry steps per case (per ADA 2025 workflow study), directly contributing to a 14% increase in daily case capacity for integrated clinics.
Conclusion: Strategic Recommendation
Bego 3D printers represent a workflow catalyst rather than a standalone device. Their open architecture, deep CAD integrations, and API-first approach (exemplified by Carejoy) deliver measurable ROI through:
- Reduction in manual intervention points (critical for labor-constrained labs)
- Future-proofing against CAD/PMS ecosystem fragmentation
- Transparent TCO modeling via competitive material markets
Implementation Advisory: For clinics/labs using mixed CAD environments (e.g., Exocad + 3Shape), Bego’s open architecture reduces integration complexity by 40% compared to closed alternatives. Prioritize deployment where material flexibility (e.g., printing both biocompatible temporaries and rigid models) is required. Closed systems remain viable only for single-vendor, single-location workflows with minimal scalability needs.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Advanced Manufacturing & Quality Control: Bego 3D Printers in China
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Executive Summary
Carejoy Digital, in strategic partnership with Bego GmbH, leverages advanced manufacturing ecosystems in China to deliver high-performance, cost-optimized 3D printing systems for digital dentistry. The production and quality control (QC) of Bego-compatible 3D printers are executed within an ISO 13485:2016-certified facility in Shanghai, ensuring medical-grade compliance, traceability, and reproducibility. This report details the manufacturing and QC processes, emphasizing sensor calibration, durability testing, and the strategic advantages of China’s digital dental equipment ecosystem.
1. Manufacturing Process: Precision Engineering in a Regulated Environment
The Bego 3D printer series—specifically the Carejoy BegoJet Pro line—is manufactured under strict adherence to ISO 13485 standards at Carejoy’s vertically integrated facility in Shanghai. The open-architecture design supports STL, PLY, and OBJ file formats, enabling seamless integration with AI-driven scanning and high-precision milling workflows.
| Stage | Process Details | Compliance & Tools |
|---|---|---|
| Component Sourcing | Laser diodes, linear encoders, and Z-stage actuators sourced from Tier-1 suppliers with ISO 13485 and IATF 16949 certification. Optical modules from German-Japanese joint ventures. | Supplier audits conducted quarterly; full material traceability via ERP system (SAP QM module). |
| Subassembly Integration | Print engine, resin vat with anti-adhesion coating, and motion control system assembled in ISO Class 7 cleanroom. Automated dispensing of optical adhesives. | ESD-safe workstations; torque-controlled tools with digital logging. |
| Final Assembly | Integration of AI-enabled calibration board, touchscreen HMI, and IoT module for remote diagnostics. Firmware flashed with version-controlled Carejoy OS 4.2. | Automated optical inspection (AOI) post-assembly; UDI labeling applied. |
2. Quality Control: Sensor Calibration & Metrological Traceability
Carejoy operates an on-site sensor calibration laboratory accredited to ISO/IEC 17025 standards, ensuring metrological accuracy across all critical subsystems.
| System | Calibration Method | Frequency & Tolerance |
|---|---|---|
| Laser Focus & Power | Beam profiler (Thorlabs BC106N-VIS) + photodiode array. Closed-loop feedback to galvo drivers. | Per unit, pre-shipment; ±0.5% power deviation, ±2µm focal precision. |
| Build Platform Flatness | Laser interferometry (ZYGO Verifire HD) across 9-point grid. | ±5µm max deviation over 140 x 80 mm build area. |
| Environmental Sensors | Temp (±0.1°C), humidity (±1.5% RH), and VOC sensors calibrated in NIST-traceable climate chamber. | Monthly recalibration; drift compensation via Carejoy Cloud AI. |
3. Durability & Reliability Testing
To ensure clinical uptime exceeding 20,000 hours, each unit undergoes accelerated life testing simulating 3 years of lab operation.
| Test Type | Protocol | Pass Criteria |
|---|---|---|
| Thermal Cycling | 100 cycles: 15°C to 40°C, 2h dwell, 1°C/min ramp. | No delamination or encoder drift >3µm. |
| Vibration & Shock | IEC 60068-2-6 (10–500 Hz, 2g) + drop test (75 cm, 6 faces). | Optical alignment maintained; no mechanical failure. |
| Print Cycle Endurance | 1,000 consecutive prints of ISO 12836 test structure (dental bridge). | Dimensional accuracy ±25µm; surface roughness Ra < 0.8 µm. |
4. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the global leader in the cost-performance optimization of digital dental systems due to the following synergistic factors:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and precision mechanics clusters (e.g., Suzhou, Shenzhen) reduces lead times and logistics costs by up to 40%.
- Advanced Automation: >75% automated assembly lines with real-time SPC (Statistical Process Control) reduce human error and increase throughput.
- Regulatory Efficiency: CFDA (NMPA) certification pathways are streamlined for ISO 13485-compliant devices, accelerating time-to-market.
- R&D Investment: Chinese tech firms reinvest ~18% of revenue into R&D, driving innovations in AI-driven calibration and predictive maintenance.
- Open Architecture Advantage: Carejoy’s support for STL/PLY/OBJ and API access enables interoperability with third-party CAD/CAM and AI scanning tools, reducing total cost of ownership.
Support & Continuous Improvement
Carejoy Digital provides:
- 24/7 Technical Remote Support via secure cloud portal with AR-assisted diagnostics.
- Monthly Software Updates including AI-driven print optimization and material profile expansion.
- Firmware Over-the-Air (FOTA) updates for field-deployed units.
Conclusion
The manufacturing and QC of Bego 3D printers in China—under Carejoy Digital’s ISO 13485-certified infrastructure—exemplify the convergence of German engineering standards and Chinese production agility. With on-site sensor calibration labs, rigorous durability protocols, and AI-enhanced support, Carejoy delivers a new benchmark in clinical reliability and cost-performance leadership for digital dental labs and clinics worldwide.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Bego 3D Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
