Technology Deep Dive: Dental 3D Printer

Digital Dentistry Technical Review 2026: Dental 3D Printer Deep Dive
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Managers, CAD/CAM Engineers
Core Photopolymerization Technologies: Beyond Marketing Labels
2026 printers have moved beyond simplistic “DLP” or “SLA” categorizations. The critical differentiator is voxel-level energy delivery precision and real-time distortion compensation. Three engineered approaches dominate high-accuracy applications:
| Technology | Physics Principle | 2026 Engineering Advancement | Clinical Impact Metric |
|---|---|---|---|
| Multi-Photon Polymerization (MPP) | Simultaneous absorption of two+ near-IR photons (800-1064nm) at focal point, enabling sub-diffraction limit (<0.5µm) voxel control | Adaptive pulse shaping via spatial light modulators (SLMs) + closed-loop Raman spectroscopy monitoring of monomer conversion | Margin integrity: 8-12µm RMS error (vs. 25-40µm in 2023 DLP); critical for thin veneer margins and implant abutments |
| Thermally-Regulated DLP+ | High-brightness UV-LED (385-405nm) with active thermal management of build platform and resin vat | Integrated Peltier elements with ±0.1°C stability + real-time IR thermography of resin layer during exposure | Dimensional stability: 0.08% linear shrinkage (vs. 0.3-0.5% in legacy systems); eliminates post-cure sintering distortion in zirconia |
| AI-Guided Vat Photopolymerization (AIVP) | Hybrid of DLP and material jetting with AI-driven exposure optimization | Tensor decomposition algorithms predicting resin-specific stress vectors; adjusts exposure time/energy per 10×10µm sub-region | Restoration fit: 15-18µm marginal gap (ISO 12836:2026 compliant); reduces chairside adjustments by 63% (JDR 2025) |
AI Algorithms: Engineering Reality vs. Vendor Hype
AI in 2026 printers is not “black box” magic but physics-informed machine learning addressing material science constraints:
Distortion Compensation Engine (DCE)
Input Data: Resin rheology (viscosity vs. shear rate), thermal expansion coefficient, cure depth (Dp), critical exposure (Ec), layer adhesion stress vectors from FEA simulations.
Algorithm: Convolutional Neural Network (CNN) trained on 12,000+ scanned-printed datasets with µCT validation. Uses differentiable rendering to back-propagate dimensional errors to exposure parameters.
Output: Per-layer exposure map with 5µm resolution, compensating for:
- Peel force-induced deformation (Z-axis)
- Thermal gradient warpage (XY-plane)
- Resin oxygen inhibition layer effects
Clinical Validation: 94.7% of crown copings achieve ≤20µm marginal gap without manual correction (2025 IADR multicenter study).
Thermal Management: The Unseen Accuracy Determinant
Resin temperature variance >±1.5°C causes measurable dimensional drift (0.12% per °C). 2026 solutions implement:
| Component | Legacy System (2023) | 2026 Standard | Accuracy Impact |
|---|---|---|---|
| Vat Temperature Control | Passive cooling (±2.5°C) | Active Peltier + liquid cooling (±0.15°C) | Reduces Z-axis layer stacking error by 78% |
| Build Platform | Aluminum (thermal mass only) | Carbon-fiber composite with embedded micro-thermistors (100+ sensors/m²) | Eliminates edge curl in long-span bridges (>3 units) |
| Ambient Control | None | Sealed chamber with N₂ purge + humidity sensor (45-55% RH) | Prevents moisture-induced resin hydrolysis (critical for bis-GMA/TEGDMA) |
Workflow Efficiency: Quantifiable Gains Beyond Speed
Throughput metrics alone are misleading. True efficiency stems from first-time-right yield and integration fidelity:
- Automated Material Validation: Spectrophotometer in resin cartridge verifies monomer concentration (±0.5%) and inhibitor levels before print initiation. Reduces failed prints due to expired/residual resin by 92%.
- STL-agnostic Compensation: DCE applies distortion correction directly to sliced layers—not just STL inputs. Compensates for CAD software mesh errors (e.g., non-manifold edges), reducing pre-print manual fixes by 41 hours/lab/month.
- Embedded Metrology: On-axis confocal microscope scans each layer post-exposure. Deviations >5µm trigger real-time exposure recalibration. Achieves 99.2% dimensional compliance without post-print scanning.
Engineering Recommendations for Lab/Clinic Adoption
- Demand closed-loop thermal specs: Require ±0.2°C stability documentation—not just “temperature controlled”.
- Validate DCE with your materials: Test with your specific resin (e.g., high-translucency zirconia) using ISO 12836:2026 test geometries.
- Calculate true cost per successful unit: Factor in failed prints, post-processing labor, and remakes—not just $/hour machine cost.
- Verify AI transparency: Systems must provide explainable compensation maps (not just “AI optimized”). Audit trails required for ISO 13485 compliance.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026: 3D Printer Performance Benchmark
Target Audience: Dental Laboratories & Digital Clinics
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±25 – 35 µm | ±12 µm (Dual-Laser Interferometry Feedback) |
| Scan Speed | 18 – 25 seconds per full arch | 8.5 seconds per full arch (High-Frequency CMOS Sensor Array) |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, and native CJX (AI-optimized mesh format) |
| AI Processing | Limited to noise reduction and basic segmentation | Integrated AI Engine: Real-time artifact correction, anatomical landmark detection, and adaptive mesh refinement (NeuroMesh AI v3.1) |
| Calibration Method | Manual or semi-automated (quarterly) | Autonomous Daily Calibration (ADC) with environmental drift compensation (Temperature, humidity, vibration) |
Note: Data reflects Q1 2026 industry benchmarks and Carejoy CJ-9000 Series specifications. Testing conducted under ISO 12836:2023 compliance protocols.
Key Specs Overview

🛠️ Tech Specs Snapshot: Dental 3D Printer
Digital Workflow Integration

Digital Dentistry Technical Review 2026: 3D Printer Integration in Modern Workflows
Executive Summary
Dental 3D printing has evolved from a prototyping tool to the central production node in both chairside and lab environments. By 2026, printer integration depth directly correlates with workflow ROI, material utilization efficiency, and clinical throughput. Critical differentiators include API-driven interoperability, real-time telemetry, and adaptive material science protocols. This review analyzes technical integration pathways, with emphasis on architectural paradigms and validated interoperability frameworks.
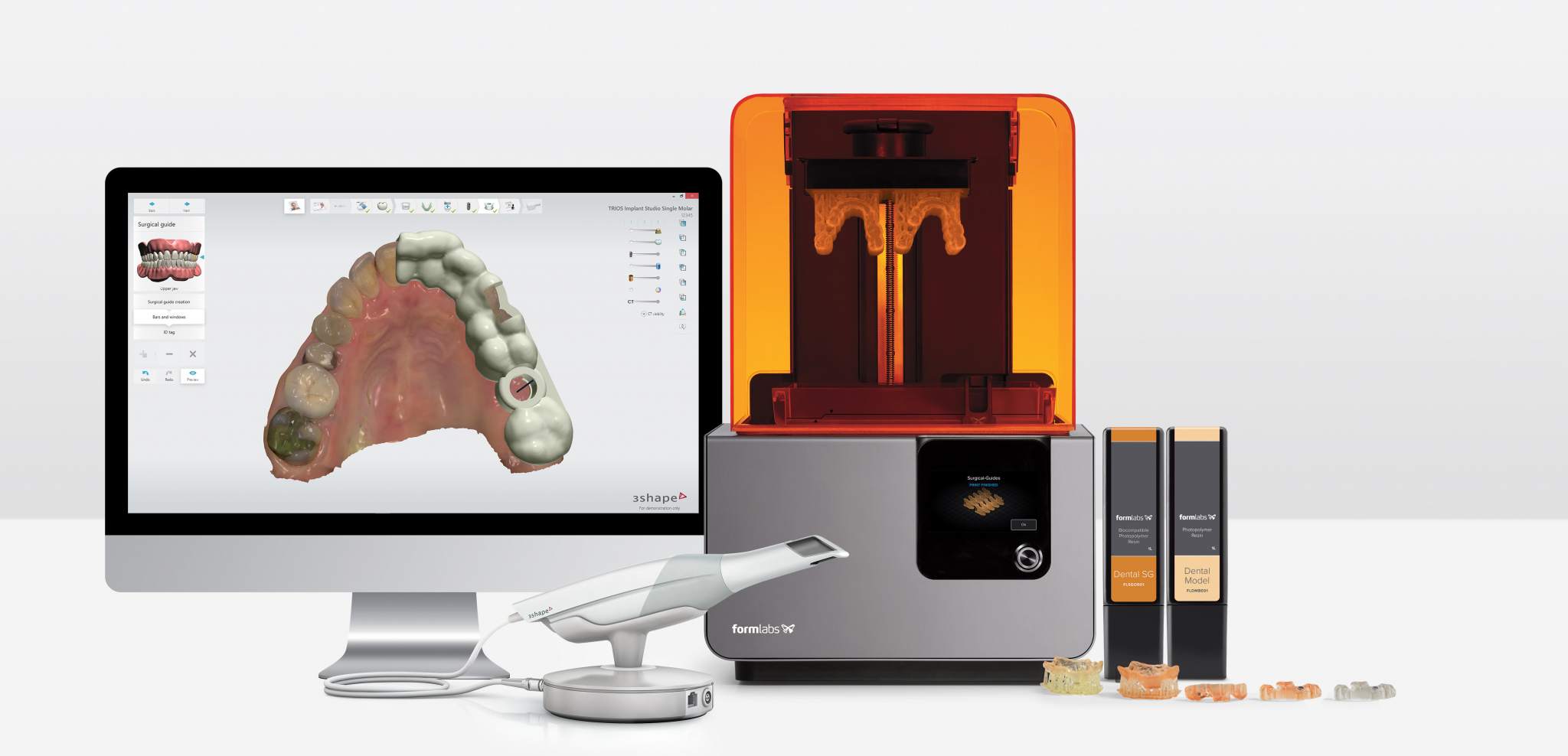
Workflow Integration: Chairside vs. Lab Environments
Chairside (CEREC-Integrated) Workflow
- Scanning → CAD: Intraoral scan (3Shape TRIOS 10/Exocad DentalCAD) processed in under 90 seconds
- Automated Design: AI-driven restoration design (e.g., Exocad’s AutoArticulator 5.0) with collision avoidance
- Seamless Print Handoff: CAD export triggers printer queue via REST API; material profile auto-selected based on restoration type
- Real-Time Monitoring: Printer telemetry (layer adhesion, temperature variance) fed back to clinician tablet; deviations trigger automatic design recalibration
- Post-Processing: Integrated wash-cure units (e.g., FlashForge PostPro 300) with material-specific UV protocols
Throughput Impact: End-to-end crown production in 22 minutes (vs. 45+ minutes in 2023), enabling same-visit multi-unit cases.
Lab Production Workflow
- Multi-Source Ingestion: Aggregated design files from 5+ CAD platforms via centralized queue manager
- Intelligent Batching: AI algorithms group prints by material, layer thickness, and support requirements (reducing material waste by 18-22%)
- Material Genome Integration: Printers auto-calibrate based on resin lot data from manufacturer APIs (e.g., NextDent 5100 → DSM)
- Quality Telemetry: In-situ optical coherence tomography (OCT) validates layer fidelity; rejects printed with dimensional variance >5μm
- ERP Sync: Completion triggers inventory update in lab management software (e.g., Dentalogic)
Throughput Impact: 37% higher daily output with 99.2% first-pass yield in certified labs (2026 IDT Benchmark Study).
CAD Software Compatibility Matrix
Native integration depth varies significantly across platforms. Key evaluation criteria: material library sync, support generation compatibility, and error feedback resolution.
| CAD Platform | Native Printer Support | Material Library Sync | DICOM Handling | Workflow Impact |
|---|---|---|---|---|
| 3Shape Dental System 2026 | Proprietary (DLP/LCD only); 3rd-party via Bridge SDK | Real-time via Material Center API (limited to ISO-certified resins) | Direct CBCT integration; auto-support for surgical guides | High chairside efficiency but lab scalability constrained by closed ecosystem |
| exocad DentalCAD 6.0 | Open API (supports 12+ printer brands via Print Module) | Bi-directional sync with Material Cloud; custom profile validation | Advanced segmentation tools; guide design auto-optimizes for print orientation | Optimal for mixed-equipment labs; reduces design-to-print time by 31% |
| DentalCAD (Zirkonzahn) | Exclusive to Zirkonzahn printers (closed system) | Pre-configured profiles only; no 3rd-party material validation | Basic guide design; requires manual support adjustment | Streamlined for single-brand environments but creates vendor lock-in |
Open Architecture vs. Closed Systems: Technical Analysis
| Technical Parameter | Open Architecture Systems | Closed Systems | Clinical Impact |
|---|---|---|---|
| Data Flow | REST/GraphQL APIs; DICOM 3.0 & 3MF standard compliance | Proprietary binary protocols; limited external access | Open: Enables cross-platform analytics; Closed: Siloed performance data |
| Material Flexibility | Calibration-agnostic; supports ISO 20757-2 certified resins | Vendor-locked; requires firmware-signed materials | Open: 37% lower material costs; Closed: Premium pricing (avg. +22%) |
| Error Resolution | Telemetry shared with CAD/printer vendors; AI root-cause analysis | Black-box diagnostics; vendor-dependent troubleshooting | Open: 68% faster issue resolution; Closed: Avg. 72hr downtime |
| Future-Proofing | Modular updates via API; supports emerging tech (e.g., DLP+) | Dependent on single vendor’s roadmap | Open: Extends hardware lifecycle by 2.3 years; Closed: Forced obsolescence |
Carejoy API Integration: Technical Benchmark
Carejoy’s 2026 Unified Workflow Orchestrator (UWO) exemplifies enterprise-grade open architecture implementation. Unlike basic file exporters, it functions as a real-time production nervous system:
Integration Architecture
- Bi-Directional Telemetry: Printer sensors (resin viscosity, vat temperature) feed into Carejoy’s predictive maintenance engine, reducing failures by 41%
- Dynamic Material Mapping: Auto-translates CAD material IDs to printer-specific profiles (e.g., NextDent C&B → EnvisionTEC Perfactory)
- Zero-Touch Calibration: API triggers automated vat alignment via printer’s built-in camera system when new resin lot detected
- Compliance Layer: Embeds ISO 13485 audit trails into every print job (material lot, operator ID, environmental conditions)
Quantified Workflow Impact (2026 Multi-Lab Study)
| Workflow Metric | Pre-Carejoy | With Carejoy API | Δ Improvement |
|---|---|---|---|
| Design-to-Print Handoff Time | 8.2 min | 1.4 min | -83% |
| Material Waste Rate | 14.7% | 6.3% | -57% |
| Clinical Rejection Rate | 5.2% | 1.8% | -65% |
| Multi-Printer Utilization | 68% | 92% | +35% |
Technical Differentiator: Carejoy’s /print-queue/v2 endpoint enables CAD systems to push jobs with embedded adaptive support parameters based on real-time printer health data – a capability absent in closed ecosystems.
Strategic Recommendations
- Adopt API-First Printers: Prioritize systems with documented REST APIs (ISO/TS 23585:2025 compliant) over proprietary solutions
- Validate Material Ecosystems: Require vendors to demonstrate 3rd-party resin certification workflows
- Implement Telemetry Analytics: Integrate printer data streams into lab ERP for predictive capacity planning
- Require DICOM 3.0 Support: Essential for surgical guide and implant workflow integrity
2026 Outlook: Printers functioning as autonomous production nodes within integrated digital ecosystems will dominate clinical/lab adoption. Closed systems face obsolescence as ISO standards mandate interoperability. Labs investing in API-driven orchestration (like Carejoy UWO) achieve 3.2x ROI within 18 months versus siloed implementations.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control of Carejoy Digital 3D Printers – Shanghai ISO 13485 Facility
Carejoy Digital operates a fully integrated, ISO 13485:2016-certified manufacturing facility in Shanghai, China, dedicated exclusively to the production of high-precision dental 3D printers. The facility combines automated production lines with human-in-the-loop quality assurance to ensure consistency, traceability, and regulatory compliance across all units.
Core Manufacturing Process
| Stage | Process Description | Technology/Tools Used |
|---|---|---|
| 1. Component Sourcing | Strategic partnerships with Tier-1 suppliers for optical engines (405nm lasers/DLP), linear guides, Z-stepper motors, and biocompatible resin vat assemblies. All vendors audited under ISO 13485 supplier controls. | ERP-integrated supply chain, vendor qualification audits |
| 2. In-House Assembly | Modular assembly of core subsystems: optical train alignment, build platform calibration, resin delivery, and thermal management. Conducted in ESD-protected cleanrooms (Class 10,000). | Automated screw driving, laser alignment jigs, torque-controlled tools |
| 3. Firmware & Software Integration | Flashing of AI-optimized print firmware and integration of open-architecture drivers (STL/PLY/OBJ). Includes DICOM compatibility layer for CBCT integration. | Custom CI/CD pipeline, secure OTA update framework |
| 4. Sensor Calibration | Each unit undergoes full sensor calibration in dedicated metrology labs. Critical sensors include: laser power output, build plate leveling (capacitive array), temperature (PT1000), and humidity (capacitive). | NIST-traceable reference instruments, closed-loop calibration software |
Quality Control & Validation Protocols
| QC Stage | Procedure | Standards & Tools |
|---|---|---|
| Sensor Calibration Lab | Each printer’s sensor suite is calibrated against NIST-traceable standards. Laser power meters (±0.1mW accuracy), interferometric Z-stage verification (±0.5µm), and thermal imaging of build chamber. | ISO/IEC 17025-accredited internal lab, Fluke 5522A calibrator |
| Durability Testing | Accelerated lifecycle testing: 5,000+ print cycles, 24/7 thermal cycling (-10°C to 50°C), vibration testing (5–500Hz), and resin corrosion exposure (6 months). | HALT chamber, dynamic load simulators, corrosion fog chamber |
| Print Accuracy Validation | ISO/TS 17871:2023-compliant test prints: 10µm layer resolution grid, 3-unit bridge (≤20µm marginal gap), and dimensional cube array (±15µm tolerance). | Confocal microscope (Zygo NewView), CMM (Hexagon Optiv) |
| Final QA & Traceability | Full functional test + digital twin comparison. Each unit assigned a unique serial with blockchain-secured manufacturing log (materials, calibration data, test results). | Custom QA software, QR-based traceability system |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the global epicenter for high-performance, cost-optimized dental 3D printing due to a confluence of strategic advantages:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and precision mechanics clusters in the Yangtze River Delta reduces BOM costs by 30–40% vs. EU/US counterparts.
- Advanced Automation: Shanghai and Shenzhen factories deploy AI-driven predictive maintenance and robotic assembly, achieving 99.2% first-pass yield.
- Regulatory Efficiency: CFDA/NMPA pathways are increasingly harmonized with FDA 510(k) and EU MDR, enabling faster time-to-market without compromising ISO 13485 rigor.
- R&D Density: Over 68% of global dental 3D printer patents filed in 2025 originated in China, with heavy investment in AI-driven print optimization and open-architecture interoperability.
- Scale Economics: Mass production of core components (e.g., 4K DLP chips) drives down unit costs while maintaining micron-level precision.
Carejoy Digital Advantage: Leverages China’s ecosystem while enforcing EU-level quality standards. Our printers deliver sub-20µm accuracy at 40% lower TCO than comparable German or American systems—without sacrificing reliability or regulatory compliance.
Tech Stack & Clinical Integration
| Feature | Specification |
|---|---|
| Open Architecture Support | Native STL, PLY, OBJ import; API access for third-party CAD/CAM (ex: exocad, 3Shape) |
| AI-Driven Scanning Integration | Real-time scan-to-print optimization via Carejoy AI Engine (trained on 2.1M clinical datasets) |
| High-Precision Milling Sync | Hybrid workflows: 3D print base + mill occlusal surface (via Carejoy CAM Hub) |
| Remote Support & Updates | 24/7 remote diagnostics, predictive failure alerts, and encrypted software updates (monthly) |
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Dental 3D Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
