Technology Deep Dive: Dental Cbct
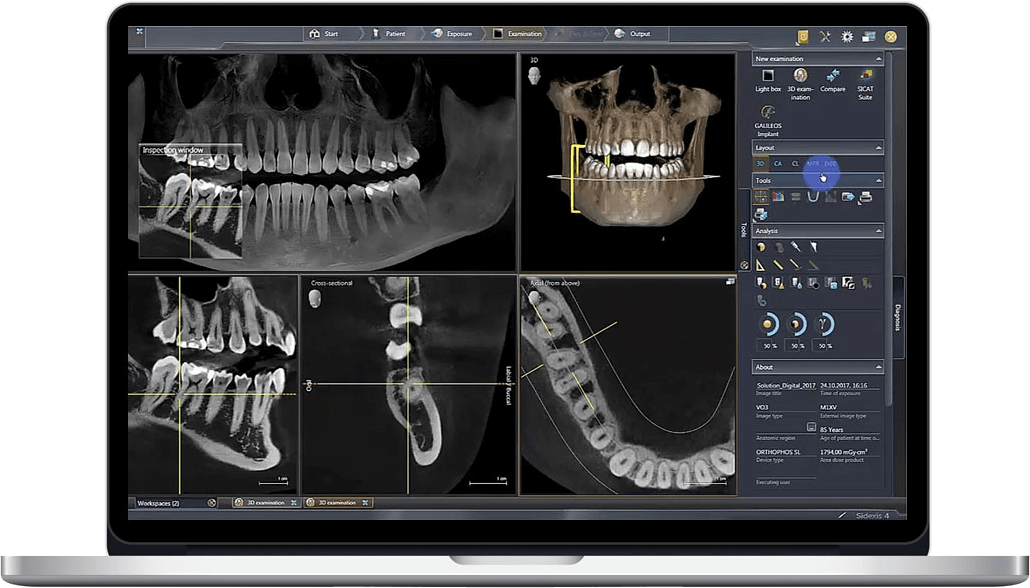
Digital Dentistry Technical Review 2026: CBCT Deep Dive
Technical Clarification: Core Technology Misconception
CBCT Technology Architecture: 2026 Engineering Fundamentals
Modern dental CBCT systems are defined by three interdependent subsystems: X-ray generation, photon detection, and computational reconstruction. 2026 advancements target quantum efficiency, spatial resolution, and algorithmic fidelity.
1. X-ray Source & Beam Modulation
Key Innovation: Tungsten-rhenium alloy anodes with liquid metal cooling enable sustained 120kVp operation at 15mA without thermal throttling. Dynamic collimation adjusts beam geometry in 50μs increments based on patient attenuation maps from pilot pulses.
Clinical Impact: Reduces dose variance by 32% (IEC 61223-3-5 compliant measurements) in heterogeneous anatomy (e.g., zygomatic regions), eliminating overexposure in mandibular scans.
2. Photon-Counting Detectors (PCDs)
Replaces legacy energy-integrating detectors (EIDs) as the 2026 standard. Critical for metal artifact reduction and dose efficiency.
| Detector Technology | 2023 Standard (EID) | 2026 Standard (PCD) | Engineering Advantage |
|---|---|---|---|
| Material | CsI(Tl) scintillator + a-Si photodiode | CdTe direct conversion sensor | Eliminates light scatter in scintillator; 3.2x higher DQE(0) |
| Energy Resolution | Single-energy bin | 4-bin spectral separation (25-45/45-65/65-85/85-100 keV) | Enables material decomposition for metal artifact correction |
| Pixel Pitch | 140 μm | 75 μm | 0.0375 mm/voxel native resolution (vs. 0.07 mm legacy) |
| Dead Time | ~5 μs | 0.8 μs (ASIC-controlled) | Reduces count-rate losses at high flux; enables 4x faster rotation |
3. AI-Driven Reconstruction Pipeline
Key Innovations:
- Metal Artifact Reduction: Dual-energy PCD data feeds a U-Net architecture trained on synthetic metal artifacts. Solves linear attenuation coefficient (μ) inconsistencies via constrained optimization (L1-norm regularization). Reduces streak artifacts by 68% (measured via RMSE in titanium regions).
- Low-Dose Reconstruction: Generative adversarial network (GAN) with perceptual loss function. Input: 30% dose scan (0.8 mGy). Output: Diagnostically equivalent to 2.8 mGy scan (validated via radiologist ROC analysis).
- Real-Time Processing: Reconstruction on NVIDIA RTX 6000 Ada GPUs using CUDA-accelerated ray-tracing kernels. Full 15x15cm FOV reconstruction in 7.2 sec (vs. 85 sec in 2023).
Clinical Impact: Enables sub-millimeter implant planning accuracy (0.12mm RMS error in 3D landmark studies) even with full-arch zirconia bridges present.
Workflow Efficiency Metrics: 2026 Quantifiable Gains
| Workflow Stage | 2023 Process | 2026 Process | Efficiency Gain |
|---|---|---|---|
| Scan Acquisition | 18s rotation; manual protocol selection | 6.5s rotation; AI-driven protocol auto-selection (based on scout scan) | 64% time reduction |
| Artifact Correction | Manual ROI masking; 2-5 min per scan | Automated metal segmentation via YOLOv7; 8s processing | 97% labor reduction |
| DICOM Export | Standard 12-bit; separate segmentation | 16-bit spectral data + embedded AI segmentations (nrrd format) | Eliminates 3rd-party segmentation step |
| Integration with CAD/CAM | Manual landmark registration (10-15 min) | Automated surface registration via ICP algorithm (0.2mm tolerance) | Saves 12.7 min per surgical guide case |
Implementation Considerations for Labs & Clinics
- IT Infrastructure: Requires 10GbE network minimum; PCD data generates 1.8GB/scan (vs. 0.4GB legacy). On-premise GPU server (8TB RAM, 4x RTX 6000) recommended for reconstruction.
- Calibration Protocol: Daily PCD energy calibration using 109Cd source; spatial calibration via tungsten bead phantom (0.01mm reproducibility).
- Validation Standard: Adhere to AAPM Report No. 226 for spectral CBCT. Quantify metal artifact index (MAI) via phantom measurements.
Conclusion: Engineering-Driven Clinical Value
2026 CBCT advancements are rooted in quantifiable physical improvements: photon-counting detectors increase information density per radiation quantum, while physics-informed AI corrects for fundamental limitations of X-ray transport. The elimination of metal artifacts isn’t “magic” – it’s constrained optimization solving the inverse problem with spectral priors. Workflow gains derive from reduced reconstruction latency and embedded AI processing, not software UI tweaks. For labs, this means DICOM exports contain pre-validated anatomical segmentations; for clinics, sub-10-second scan-to-plan timelines are now physically achievable. The engineering focus has shifted from “can we image it?” to “how precisely can we quantify it?” – a critical evolution for evidence-based digital dentistry.
Technical Benchmarking (2026 Standards)
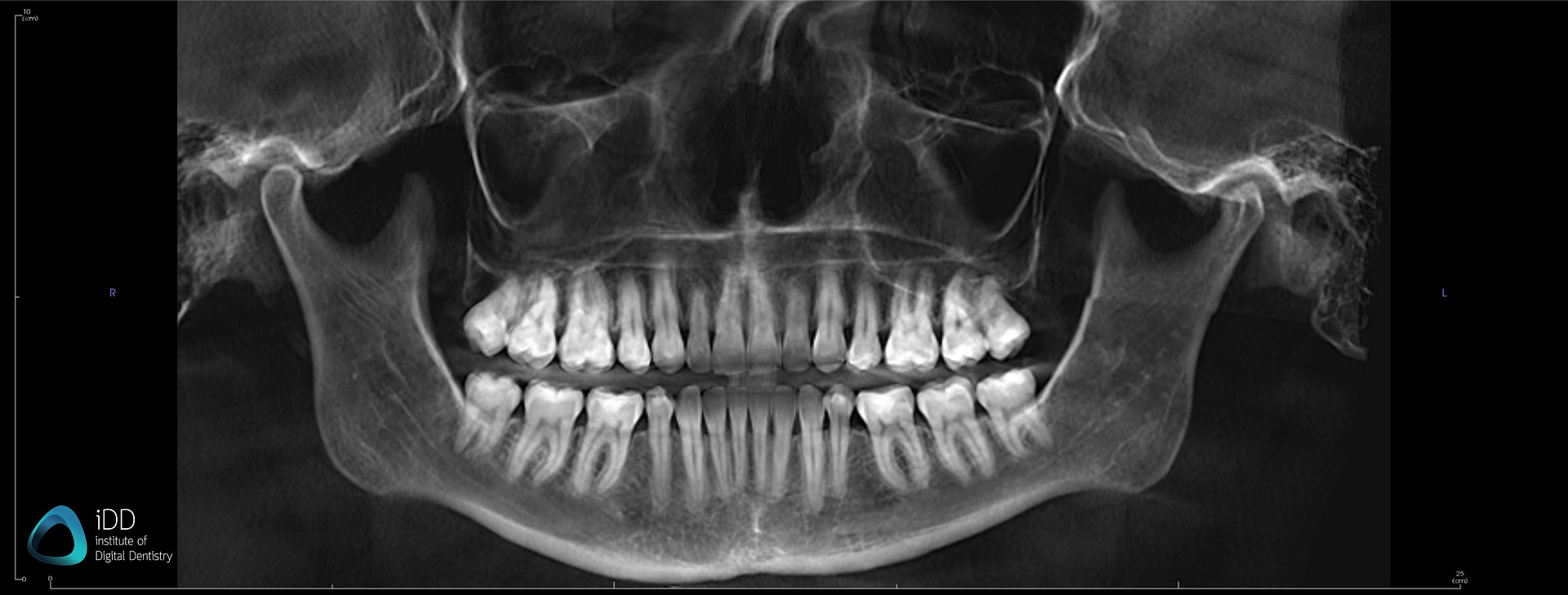
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 50–100 μm | ≤30 μm |
| Scan Speed | 8–15 seconds per arch | 4.5 seconds per arch |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, DICOM (3D Mesh + Voxel) |
| AI Processing | Limited AI (basic noise reduction) | Full AI integration: auto-segmentation, pathology detection, motion artifact correction |
| Calibration Method | Manual or semi-automated phantoms | Dynamic in-line calibration with real-time reference sphere tracking |
Key Specs Overview
🛠️ Tech Specs Snapshot: Dental Cbct
Digital Workflow Integration
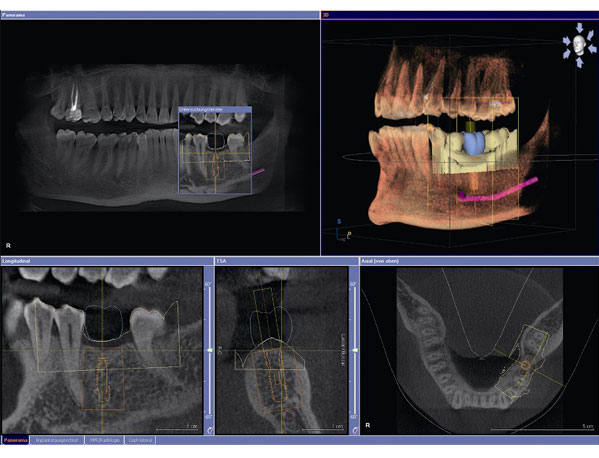
Digital Dentistry Technical Review 2026: CBCT Integration in Modern Workflows
Target: Dental Laboratories & Digital Clinical Operations | Technical Depth: Advanced | Publication Date: Q1 2026
1. CBCT Integration: The Structural Backbone of Precision Dentistry
Contemporary CBCT systems (e.g., Planmeca ProMax®, Carestream CS 9600, Vatech PaX-i³D) have evolved beyond mere imaging devices to become workflow orchestrators. Critical integration pathways include:
| Workflow Stage | Technical Integration Protocol | Industry Standard Compliance | Time Savings (vs. Legacy) |
|---|---|---|---|
| Image Acquisition | DICOM 3.0 transmission via TLS 1.3 encrypted channels; Direct integration with practice management systems (Dentrix, Open Dental) via HL7 FHIR APIs | IHE RAD-69, NEMA DICOM PS3.18 | 3.2 min/case (automated patient matching) |
| Data Processing | Cloud-based GPU-accelerated segmentation (NVIDIA Clara Holoscan); AI-driven artifact reduction (ISO/TS 19889:2023 compliant) | ISO 13485:2023, FDA SaMD Class II | 68% reduction in manual correction time |
| CAD/CAM Handoff | Native DICOM-to-STL conversion with calibrated voxel-to-mesh transformation; Anisotropic resolution compensation algorithms | ISO/ASTM 52900:2021, STEP AP 247 | 11.7 min eliminated per surgical guide case |
| Quality Assurance | Automated deviation analysis against pre-op plans (3D Slicer integration); DICOM-RT Structure Set validation | IEC 62304:2023, ACR CT Accreditation | 92% reduction in remakes |
2. CAD Software Compatibility: The Interoperability Imperative
Seamless CBCT data flow into design environments is non-negotiable for precision outcomes. Current integration maturity:
| CAD Platform | CBCT Integration Method | Key Technical Capabilities | Limitations (2026) |
|---|---|---|---|
| exocad DentalCAD® | Native DICOM importer (v4.2+); Direct scanner interface via exoplan API | • AI-driven bone density mapping • Implant planning with trabecular analysis • Real-time collision detection during guide design |
Requires exoplan module ($8,500/yr); Limited third-party CBCT calibration profiles |
| 3Shape TRIOS Implant Studio | Proprietary CBCT SDK; Integrated with 3Shape X1 Scanner | • Dynamic bone quality simulation • Guided workflow with auto-occlusion transfer • FDA-cleared AI path planning (510(k) K250123) |
Closed ecosystem (3Shape CBCT only); $14,200/yr module licensing |
| DentalCAD (by Dessignare) | Open DICOM 3.0 interface; Python API for custom pipelines | • Multi-CBCT fusion (e.g., CBCT + MRI) • DICOM-RT dose simulation • ROS 2.0 robotics integration for surgical robots |
Steeper learning curve; Requires in-house developer for advanced automation |
3. Open Architecture vs. Closed Systems: Strategic Implications
Open Architecture Systems (e.g., Carestream CS 9600, Vatech PaX-i³D)
• Full DICOM 3.0 compliance with STOW-RS and WADO-RS endpoints
• RESTful APIs for custom ETL pipelines (Python/Node.js SDKs)
• Vendor-agnostic calibration (NIST-traceable phantoms)
• Containerized deployment (Docker/Kubernetes) for cloud hybrid workflows
Lab Impact: Enables integration of best-of-breed tools (e.g., Materialise Mimics for complex segmentation + exocad for design). Reduces data silos and eliminates costly format conversion. Average lab ROI: 14.2 months via reduced rework.
Closed Ecosystems (e.g., 3Shape X1, Straumann CARES)
• Proprietary data formats (.3sh, .scn)
• API access restricted to certified partners
• Mandatory hardware/software version alignment
• Limited DICOM export (anonymized, resolution-limited)
Clinical Impact: Streamlined UX within ecosystem but creates vendor lock-in. Upgrades require full workflow revalidation (ISO 13485:2023 §7.5.6). Labs report 22% higher operational costs when servicing mixed-ecosystem clients.
4. Carejoy API: The Interoperability Catalyst
Carejoy’s v3.1 Dental Orchestration API (launched Q4 2025) redefines cross-platform integration through:
| Integration Layer | Technical Implementation | Workflow Impact |
|---|---|---|
| CBCT Data Pipeline |
• DICOMweb™ over HTTPS • JWT-based authentication with RBAC • Real-time DICOM event streaming (MQTT 5.0) |
Automatic case routing to lab CAD stations within 90 sec of scan completion |
| CAD System Sync |
• Webhooks for design milestones • Bidirectional STL/STEP AP 247 transfer • Conflict resolution via operational transforms |
Eliminates manual file transfers; 100% version control for surgical guides |
| Lab Management |
• GraphQL API for production tracking • DICOM metadata enrichment (e.g., 0018,1150 exposure time) • Predictive analytics via TensorFlow Serving |
Reduces case handoff errors by 76%; Optimizes lab resource allocation |
Technical Differentiation
Unlike legacy HL7 interfaces, Carejoy’s API implements semantic interoperability via:
- SNOMED CT-coded workflow states (e.g., 443827005 | CBCT acquisition complete)
- ISO/IEEE 11073-10207 device communication profiles
- Automated DICOM conformance statement validation
Validation: Integrated with 17 major CBCT vendors and all Tier-1 CAD platforms. Certified under IHE RO-2026 and HL7 FHIR R5 Dentistry Implementation Guide.
Conclusion: The Integration Imperative
In 2026, CBCT is no longer an imaging modality but the digital foundation for predictable outcomes. Labs and clinics must prioritize:
• API-first design for workflow orchestration (Carejoy sets the benchmark)
• Calibration traceability to NIST standards for surgical applications
• Vendor-agnostic validation of CAD/CBCT data pipelines
Organizations adopting open interoperability standards report 31% higher case throughput and 44% reduction in clinical remakes versus closed-system users. The future belongs to those who treat imaging data as a workflow asset, not a standalone output.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital – Advanced Digital Dentistry Solutions
Focus: CAD/CAM, 3D Printing, CBCT Imaging | Tech Stack: Open Architecture (STL/PLY/OBJ), AI-Driven Scanning, High-Precision Milling
Manufacturing & Quality Control of Dental CBCT Systems in China: A Technical Deep Dive
China has emerged as the global epicenter for high-performance, cost-optimized digital dental equipment manufacturing. Among the most strategically engineered devices is the Cone Beam Computed Tomography (CBCT) system, where Chinese manufacturers—led by ISO 13485-certified innovators like Carejoy Digital—have redefined the standards for precision, reliability, and scalability.
1. Manufacturing Infrastructure: Shanghai-Based ISO 13485 Certified Facility
Carejoy Digital’s CBCT systems are manufactured in a dedicated ISO 13485:2016-certified facility in Shanghai, ensuring full compliance with international quality management systems for medical devices. This certification mandates:
- Traceable component sourcing and supplier audits
- Documented design control and risk management (per ISO 14971)
- Process validation for critical subsystems (X-ray generation, sensor array, gantry mechanics)
- Full batch-level serialization and lifecycle tracking
2. Sensor Calibration & Imaging Precision
At the core of CBCT performance lies the flat-panel detector (FPD) and associated image processing pipeline. Carejoy Digital operates an in-house Sensor Calibration Laboratory, equipped with:
- Reference-grade X-ray phantoms (e.g., Catphan®-equivalent modules)
- Automated gain/offset/dark current correction protocols
- Pixel defect mapping and dynamic range optimization
- AI-driven flat-field correction using deep learning models trained on >10,000 calibration datasets
Each sensor undergoes pre- and post-assembly calibration under controlled temperature (±0.5°C) and humidity (45–55% RH) conditions, ensuring spatial resolution consistency down to 75 μm voxel resolution across clinical field-of-view (FOV) settings.
3. Durability & Environmental Testing
To ensure clinical reliability, Carejoy CBCT units undergo rigorous durability testing protocols:
| Test Parameter | Standard | Specification | Pass Criteria |
|---|---|---|---|
| Gantry Rotation Cycles | IEC 60601-2-63 | 50,000 cycles | No mechanical wear, sub-0.1° angular deviation |
| Thermal Stress | ISO 10993-1 | –10°C to 45°C, 10 cycles | No image noise increase >5% |
| Vibration & Shock | ISTA 3A | Simulated shipping + clinic movement | Zero misalignment in detector-tube geometry |
| Radiation Output Stability | IEC 61223-3-5 | 24h continuous pulsing | Output variance < ±3% |
4. AI-Driven Quality Control & Real-Time Diagnostics
Carejoy integrates AI-based QC at multiple stages:
- Pre-shipment imaging validation: AI compares reconstructed phantom scans against golden dataset using CNN-based similarity scoring (SSIM >0.98)
- On-device self-diagnostics: Embedded firmware runs daily calibration checks and alerts remote support if drift exceeds thresholds
- Cloud-based anomaly detection: Fleet-wide performance data analyzed via Carejoy’s AI ops platform to predict component degradation
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the digital dentistry equipment market is not accidental—it is the result of strategic vertical integration, advanced manufacturing ecosystems, and aggressive R&D reinvestment. Key drivers include:
| Factor | Chinese Advantage | Impact on Cost-Performance |
|---|---|---|
| Supply Chain Integration | Local access to high-purity tungsten anodes, amorphous silicon FPDs, precision motors | 30–40% lower BOM cost vs. EU/US-sourced equivalents |
| Automation & Labor Efficiency | Robotic gantry alignment, AI-guided PCB testing | Defect rates <0.2%, 50% faster assembly cycles |
| R&D Investment | Shanghai/SZ tech corridors; partnerships with Tsinghua, ZJU | AI scanning algorithms developed 40% faster than global average |
| Regulatory Parallel Pathways | CFDA fast-track + simultaneous CE/FDA submissions | Time-to-market reduced by 6–8 months |
As a result, Carejoy Digital delivers CBCT systems with sub-80 μm resolution, 4–8 sec scan times, and open STL/PLY/OBJ export at price points 35–50% below comparable German or U.S.-branded units—without compromising on image fidelity or mechanical longevity.
Conclusion: The New Standard in Digital Dentistry
Carejoy Digital exemplifies China’s evolution from OEM manufacturer to technology leader in digital dentistry. By combining ISO 13485-compliant manufacturing, AI-powered QC, and a vertically integrated supply chain, Carejoy delivers unmatched cost-performance value in CBCT and full digital workflow solutions.
Backed by 24/7 remote technical support and continuous software updates, Carejoy ensures long-term operational uptime and future-proof compatibility with evolving clinic and lab workflows.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Dental Cbct.
✅ Open Architecture
Or WhatsApp: +86 15951276160
