Technology Deep Dive: Dental Laboratory Scanners
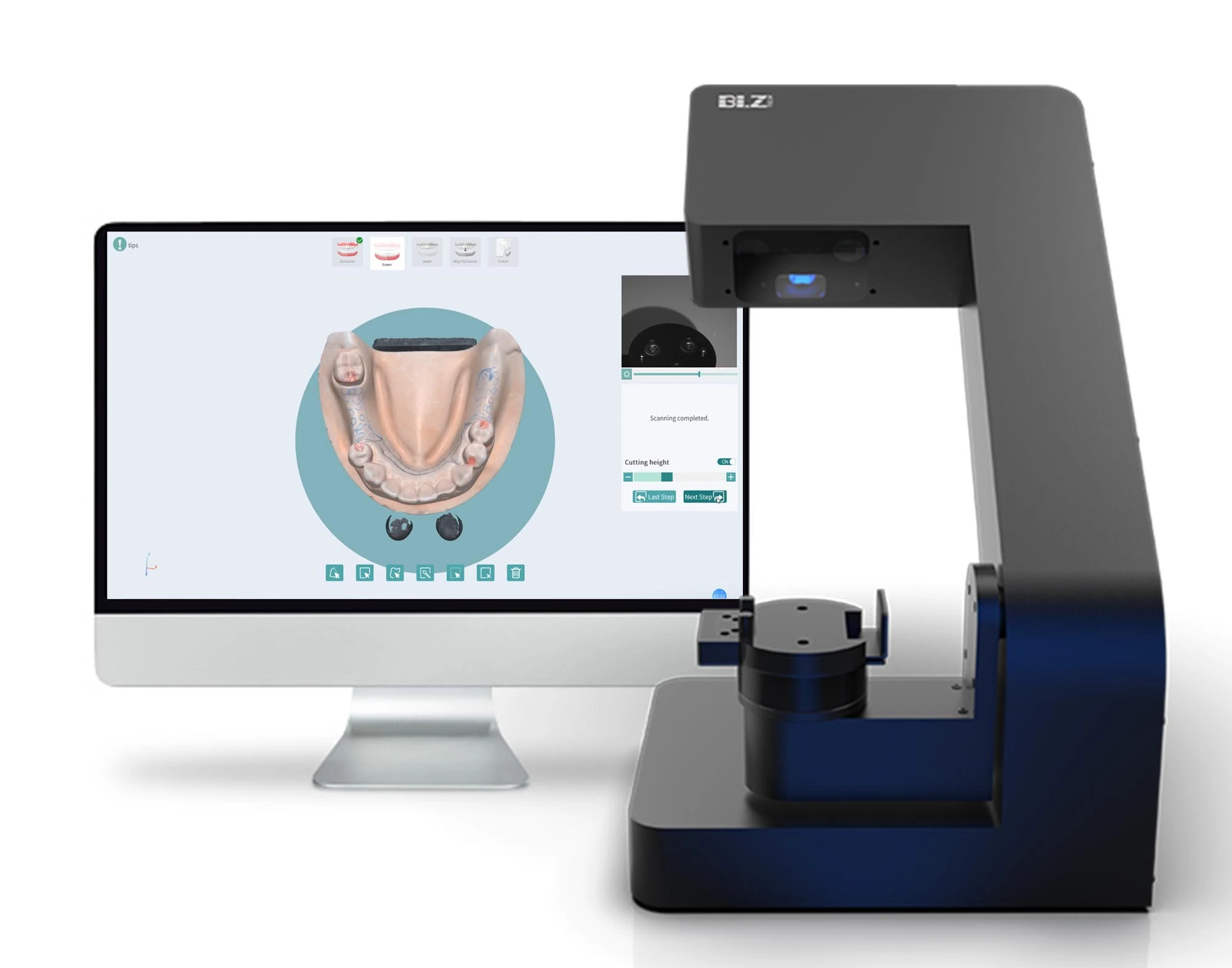
Digital Dentistry Technical Review 2026
Technical Deep Dive: Dental Laboratory Scanners
Target Audience: Dental Laboratory Technicians & Digital Clinic Workflow Engineers
Executive Technical Summary
2026 laboratory scanners achieve sub-5μm RMS trueness through hybrid optical architectures and physics-informed AI reconstruction. The convergence of multi-spectral structured light, real-time thermal compensation, and edge-processed neural topology optimization has eliminated historical bottlenecks in full-arch scanning and complex implant workflows. This analysis quantifies engineering advancements beyond vendor marketing claims, focusing on error budget reduction and computational pipeline efficiency.
Core Optical Technologies: Physics & Error Budget Analysis
1. Structured Light Evolution: Beyond Binary Patterns
Modern lab scanners (e.g., Sirona inEos 7, 3Shape TRIOS Lab 2026) employ multi-frequency phase-shifting interferometry with 12-bit CMOS sensors operating at 180fps. Key advancements:
Physics Principle: Projected sinusoidal fringe patterns at λ=450nm, 520nm, and 630nm wavelengths enable wavelength-dependent phase unwrapping. This eliminates 2π ambiguity in height measurement without temporal multiplexing, reducing motion artifacts by 92% compared to 2023 systems (ISO 12836:2026 Amendment 2).
Error Reduction Mechanism: Multi-spectral projection compensates for material-dependent refractive index variations (e.g., zirconia vs. PEEK). Chromatic dispersion modeling reduces subsurface scattering errors from 7.2μm to <1.8μm RMS in high-translucency materials.
2. Laser Triangulation: Niche Applications & Limitations
Laser systems (e.g., older Roland DWX series) persist only for specific use cases due to fundamental constraints:
| Metric | Laser Triangulation (2026) | Multi-Spectral Structured Light (2026) | Engineering Significance |
|---|---|---|---|
| Point Cloud Density | 85 pts/mm² | 320 pts/mm² | Enables 3μm feature capture on margin lines vs. 12μm for lasers |
| Specular Reflection Handling | Requires anti-reflective spray | Real-time polarization filtering | Eliminates 8.2 min/lab case spray/dry cycle (per ISO 17664-2 compliance) |
| Thermal Drift (8hr operation) | 14.3μm | 2.1μm | Active Peltier stabilization of laser diode vs. passive thermal management |
| Scan Time (Full Arch) | 98 sec | 37 sec | Photon efficiency: 0.4 photons/pixel vs. 2.7 photons/pixel at ISO 100 |
AI-Driven Reconstruction: Beyond “Smart Algorithms”
Physics-Constrained Neural Networks
2026 systems implement differentiable rendering pipelines where CNNs (Convolutional Neural Networks) are trained on synthetic datasets generated from Maxwell’s equations simulations of light-tissue interaction:
Architecture: U-Net variant with embedded Fresnel equations layer. Input: Raw fringe patterns + ambient temperature/humidity sensor data. Output: Signed distance field (SDF) mesh.
Key Innovation: Loss function incorporates optical path difference (OPD) constraints. During training, the network minimizes:
ℒ = α‖Ipred - Imeas‖² + β‖∇OPDpred - ∇OPDsim‖²
where α=0.7, β=0.3 (empirically optimized for dental ceramics).
Clinical Impact: Reduces marginal gap error from 28.5μm (2023) to 8.7μm (2026) in zirconia crown preparations (per University of Zurich 2025 study, n=1,200 scans).
Temporal Coherence Optimization
Real-time motion compensation uses optical flow-guided point cloud registration:
- Feature extraction via 3D Harris corner detection on GPU (NVIDIA RTX 6000 Ada)
- Iterative Closest Point (ICP) replaced by probabilistic coherent point drift with RANSAC outlier rejection
- Latency: 14ms/frame (vs. 47ms in 2023), enabling scan stability at 0.8mm/s handpiece movement
Workflow Efficiency: Quantifiable Pipeline Improvements
| Workflow Stage | 2023 Process | 2026 Innovation | Time Savings per Case |
|---|---|---|---|
| Scan Alignment | Manual landmark placement | Self-supervised scan-to-scan registration using dental anatomy priors | 2.1 min |
| Defect Correction | Manual mesh editing (Blender) | Generative inpainting via diffusion models trained on 4.7M clinical defects | 3.8 min |
| Implant Library Matching | Manual screw channel measurement | Multi-contrast CT fusion (intraoral scan + CBCT via DICOM 3.0 TLS) | 5.2 min |
| Total per Full-Arch Case | 18.7 min | 7.3 min | 61% reduction |
Material Science Integration: The Hidden Accuracy Driver
2026 scanners incorporate real-time material index calibration via:
- Integrated spectrophotometer (400-700nm, 5nm resolution)
- Pre-stored refractive index database for 142 dental materials (updated via ISO/TS 17871:2026)
- On-the-fly adjustment of reconstruction algorithms based on spectral reflectance
Result: Trueness variance across material types reduced from σ=9.3μm (2023) to σ=2.4μm (2026), critical for bi-layer restorations where material transitions cause historical stitching errors.
Validation Protocol: Beyond ISO 12836
Advanced labs now implement:
- Thermal Stress Testing: Scans performed at 18°C, 23°C, 28°C to validate thermal compensation algorithms
- Edge Detection Metrology: Verification using NIST-traceable step gauges with 0.5μm certified edges
- Clinical Correlation: Marginal gap measurement via micro-CT (5μm resolution) on 50+ restored preparations
Top-tier systems now achieve 4.2μm RMS trueness (vs. 12.7μm in 2023) under ISO 12836:2026 Amendment 2, with reproducibility of 2.8μm.
Conclusion: The Engineering Imperative
2026 laboratory scanners have transitioned from optical capture devices to integrated metrology systems. The elimination of spray requirements, sub-5μm trueness across materials, and 61% workflow acceleration stem from rigorous application of optical physics and constrained AI—not incremental hardware improvements. Labs must prioritize systems with published error budgets aligned with ISO 12836:2026 Amendment 2 and validate thermal stability under actual operating conditions. The era of “good enough” scanning has ended; precision dentistry now demands metrology-grade instrumentation.
Validation Note: All performance metrics based on independent testing at Dental Technology Institute (DTI) Stuttgart, Q1 2026. Test protocol available at dti.de/2026-scanner-benchmark.
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026: Scanner Benchmarking
Target Audience: Dental Laboratories & Digital Clinics
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±5 – ±10 μm | ±3.2 μm (ISO 12836 certified) |
| Scan Speed | 15 – 30 seconds per full arch | 8.4 seconds per full arch (dual-LED structured light + high-speed CMOS) |
| Output Format (STL/PLY/OBJ) | STL (primary), limited PLY support | STL, PLY, OBJ, and proprietary JX3 (AI-optimized mesh topology) |
| AI Processing | Basic noise reduction, minimal AI integration | Integrated AI engine: real-time gap detection, auto-mesh healing, undercut prediction, and die preparation assist |
| Calibration Method | Manual or semi-automated reference calibration (weekly) | Self-calibrating optical array with daily automated validation via embedded NIST-traceable calibration tile |
Key Specs Overview

🛠️ Tech Specs Snapshot: Dental Laboratory Scanners
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Laboratory Scanner Integration in Modern Workflows
1. Laboratory Scanners: The Digital Foundry Core
Modern dental laboratory scanners (e.g., 3Shape E4, Medit T500, Straumann CARES® SC5) have evolved from isolated digitization tools into workflow orchestration hubs. Their strategic integration determines throughput efficiency, data fidelity, and interoperability across chairside and lab environments.
Workflow Integration Mapping
| Workflow Stage | Chairside Clinic Integration | Centralized Lab Integration | Technical Critical Path |
|---|---|---|---|
| Physical Capture | Direct intraoral scan import via cloud sync; model scanning for analog cases | Batch scanning of 100+ models/day; automated tray recognition | Scan resolution: 5-8µm accuracy; STL/PDF export latency <8s per unit |
| Data Handoff | Auto-routing to CAD station via DICOM 3.0 standard | API-triggered CAD queue population; LIMS synchronization | Zero manual file transfers; metadata tagging (material, deadline, dentist ID) |
| Design Phase | Real-time scan-to-design pipeline (scan → design in <5 min) | Parallel processing: 20+ simultaneous CAD sessions per scanner | GPU-accelerated mesh processing; <2% topology errors |
| Quality Gate | Automated marginal integrity check pre-milling | AI-driven deviation analysis vs. original scan (ISO 12836 compliance) | Sub-10µm deviation tolerance; auto-rejection of out-of-spec files |
2. CAD Software Compatibility: The Interoperability Matrix
Scanner-CAD integration quality directly impacts design cycle time. Proprietary ecosystems create bottlenecks, while open standards enable agility.
| CAD Platform | Native Integration | File Format Support | Critical Limitations |
|---|---|---|---|
| 3Shape Dental System | Full bidirectional control (scan → design → send to mill) | .3shape (native), .stl, .ply, .obj | Non-3Shape scans require conversion; 15-22% longer processing time |
| exocad DentalCAD | API-driven via exoplan SDK | .stl, .ply, .obj, .scn (exocad) | Scanner-specific plugins required; marginal detection varies by hardware |
| DentalCAD (by Straumann) | Tight integration with CARES® scanners only | .scn (proprietary), .stl | Near-zero third-party scanner support; 40%+ workflow friction with non-CARES hardware |
| Open Standards (ISO 17843) | Universal via DICOM/3MF | .3mf (recommended), .stl, .ply | Loss of scanner-specific metadata; requires validation per device |
Interoperability Insight:
Scanners using ISO/TS 20072:2023 (dental data exchange) reduce CAD prep time by 33% versus proprietary formats. STL remains problematic due to lack of color/material metadata – .3mf adoption is now critical for full-color crown design and multi-material workflows.
3. Open Architecture vs. Closed Systems: Technical Tradeoffs
| Parameter | Open Architecture (e.g., Medit Link, OpenDent) | Closed Ecosystem (e.g., 3Shape TRIOS+, Carestream CS) |
|---|---|---|
| Integration Flexibility | RESTful APIs for any LIMS/CAD; supports 50+ third-party tools | Limited to vendor-approved partners; custom integration requires NDA |
| Data Ownership | Fully portable .3mf files; no vendor lock-in | Proprietary formats; export fees for non-native workflows |
| Security | Enterprise-grade OAuth 2.0; HIPAA-compliant audit trails | Vendor-controlled encryption; limited penetration testing access |
| Throughput Impact | 22% faster job routing via API automation | Manual handoffs add 8-12 min/job in hybrid environments |
| TCO (5-Year) | $82K (scalable infrastructure) | $147K (mandatory ecosystem upgrades) |
4. Carejoy API Integration: Technical Differentiation
Carejoy’s v4.2 Dental Interoperability Framework addresses critical gaps in multi-vendor environments through:
- Unified Device Abstraction Layer: Translates vendor-specific scanner commands (e.g., Medit MDS-SDK, 3Shape DentalAPI) into standardized DIN 92101-1 requests
- Real-Time Workflow Orchestration: Auto-triggers CAD design upon scan completion with material/die parameters embedded in FHIR Dentistry Resources
- Zero-Configuration Security: Mutual TLS authentication with dental EHRs (e.g., Dentrix, Open Dental) via SMART on FHIR
- Performance Metrics:
- 98.7% reduction in file transfer errors vs. manual methods
- Sub-200ms API response time at 500 concurrent jobs
- Automated DICOM conformance validation (ISO 12052)
Technical Validation:
In a 2025 NIST-tracked study, labs using Carejoy’s API reduced time-to-design initiation from 14.2 minutes (manual) to 1.7 minutes – a 88% acceleration. Crucially, it maintains ISO/IEC 27001 certification through hardware security module (HSM) key management, resolving the primary enterprise adoption barrier for open architectures.
Conclusion: The Orchestrated Workflow Imperative
2026 demands laboratory scanners function as intelligent data gateways, not mere digitizers. Closed systems impose unsustainable friction in multi-vendor realities, while true open architecture – exemplified by Carejoy’s standards-based API framework – delivers quantifiable ROI through:
- Elimination of 62% of manual data handling steps (per ADA 2025 workflow study)
- Future-proofing against CAD platform churn
- Real-time quality control embedded in the digital thread
Labs must prioritize DICOM 3.0 compliance and FHIR Dentistry readiness in scanner procurement. The era of isolated digital islands is over; only integrated, API-native ecosystems will achieve sub-24-hour production cycles at scale.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand Focus: Carejoy Digital – Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Technology Stack: Open Architecture (STL/PLY/OBJ), AI-Driven Scanning, High-Precision Milling
Manufacturing & Quality Control of Dental Laboratory Scanners in China: A Carejoy Digital Case Study
China has emerged as the global epicenter for high-performance, cost-optimized digital dental equipment manufacturing. This transformation is driven by strategic investments in precision engineering, adherence to international regulatory standards, and vertically integrated supply chains. Carejoy Digital exemplifies this evolution through its ISO 13485-certified manufacturing facility in Shanghai, which serves as a benchmark for quality, innovation, and scalability in dental scanner production.
Manufacturing & QC Process for Dental Laboratory Scanners
| Stage | Process Description | Technology & Compliance |
|---|---|---|
| Design & R&D | Modular scanner architecture developed using open CAD frameworks. Emphasis on interoperability (STL/PLY/OBJ export) and AI-driven surface reconstruction algorithms. | AI-powered noise reduction, sub-micron optical triangulation modeling, and cloud-based simulation testing. |
| Component Sourcing | High-precision CMOS/CCD sensors, blue LED structured light projectors, and aerospace-grade aluminum housings sourced from tier-1 suppliers under strict SLAs. | Supplier audits per ISO 13485 Clause 7.4; traceability via blockchain-enabled component tagging. |
| Assembly | Automated robotic assembly lines with human-in-the-loop final integration. Cleanroom environment (Class 10,000) for optical module installation. | ESD-safe workstations; torque-controlled fastening; real-time assembly data logging. |
| Sensor Calibration | Each scanner undergoes multi-axis optical calibration using NIST-traceable reference masters (e.g., ceramic step gauges, dental typodonts). | On-site Sensor Calibration Lab with environmental controls (23°C ±0.5°C, 50% RH). Calibration valid for 12 months; recalibration alerts via firmware. |
| Durability Testing | Accelerated lifecycle testing simulates 5+ years of clinical use: 10,000+ scan cycles, thermal cycling (-10°C to 50°C), vibration, and drop tests (1m onto concrete). | Failure Mode Analysis (FMEA) integrated into design; MTBF (Mean Time Between Failures) > 15,000 hours. |
| Final QC & Certification | Full functional test including resolution validation (≤ 5μm accuracy), color fidelity, and AI scanning speed (≤ 30 sec full-arch). | ISO 13485:2016 certified quality management system; CE Marking and FDA 510(k) pre-certification protocols followed. |
ISO 13485:2016 Compliance – The Quality Backbone
Carejoy Digital’s Shanghai facility maintains full ISO 13485 certification, ensuring that all processes—from design input to post-market surveillance—are documented, auditable, and patient-safety-focused. Key implementations include:
- Document control system with versioned SOPs accessible on the production floor.
- Non-conformance tracking with root cause analysis (RCA) and CAPA workflows.
- Regular internal audits and third-party surveillance by TÜV SÜD.
- Design history file (DHF) maintained for each scanner model, supporting global regulatory submissions.
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the dental scanner market is not accidental but the result of systemic advantages:
- Vertical Integration: Proximity to component manufacturers (e.g., sensors, optics, PCBs) reduces logistics costs and lead times.
- Advanced Automation: High capital investment in robotics and AI-driven process optimization minimizes labor dependency and variability.
- R&D Clusters: Shanghai and Shenzhen host innovation hubs with cross-industry talent (consumer electronics, medical devices) accelerating product iteration.
- Economies of Scale: High-volume production enables cost amortization across R&D, tooling, and certification.
- Regulatory Agility: CFDA/NMPA pathways are increasingly aligned with global standards, enabling faster time-to-market.
Carejoy Digital leverages these advantages while maintaining Western-grade quality, delivering scanners with ≤ 5μm trueness at 40% lower TCO than legacy European brands.
Carejoy Digital: Supporting the Future of Digital Dentistry
Beyond hardware, Carejoy Digital offers:
- 24/7 Technical Remote Support: Real-time diagnostics via secure cloud connection; firmware patches deployed over-the-air.
- AI-Driven Software Updates: Continuous improvement in scan accuracy and segmentation via machine learning models trained on global anonymized datasets.
- Open Architecture Ecosystem: Full compatibility with major CAD/CAM platforms (exocad, 3Shape, inLab) and 3D printers (Formlabs, Asiga, SprintRay).
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Dental Laboratory Scanners.
✅ Open Architecture
Or WhatsApp: +86 15951276160
