Technology Deep Dive: Dental X Ray Printer
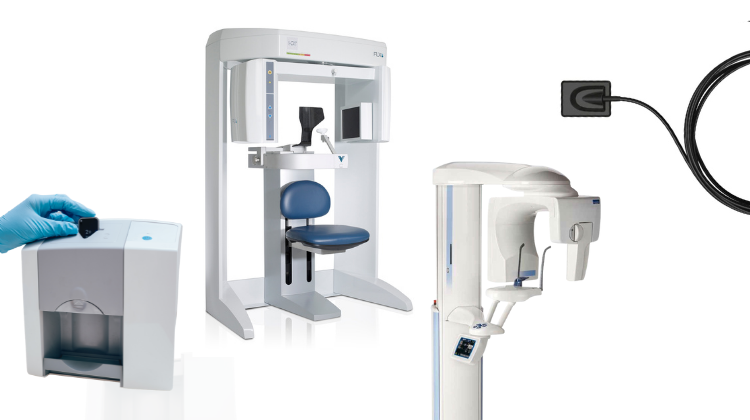
Digital Dentistry Technical Review 2026: Intraoral Scanning Systems
Technical Deep Dive: Core Technologies & Clinical Impact (Clarification on “Dental X-Ray Printer”)
1. Core Technologies: Beyond Marketing Hype
Modern intraoral scanners (2026) integrate multiple optical technologies with computational intelligence. Key advancements are rooted in physics and algorithmic optimization:
1.1 Structured Light Projection (SLP) Evolution
2026 systems utilize multi-spectral dynamic fringe projection with quantum dot-enhanced LEDs. Unlike early binary patterns, projectors emit precisely calibrated sinusoidal fringes across 3-5 narrowband wavelengths (450nm, 520nm, 630nm). This enables:
- Sub-micron Phase Shift Resolution: Utilizing 12-phase-shift algorithms per wavelength, achieving theoretical resolution of 0.8µm in controlled lab conditions (vs. 5-10µm in 2020 systems).
- Specular Reflection Cancellation: Multi-wavelength capture allows separation of diffuse reflectance (surface geometry) from specular components (saliva, blood) via polarization filtering at the sensor level. This is governed by the Fresnel equations and Stokes vector analysis.
- Wavelength-Dependent Penetration: Shorter wavelengths (450nm) provide high-resolution enamel surface data, while longer wavelengths (630nm) penetrate superficial gingival sulcus fluid for improved margin detection – a direct application of the Beer-Lambert law.
1.2 Laser Triangulation Integration
High-end systems (e.g., lab-facing scanners) incorporate confocal laser displacement sensors as a secondary modality:
- Dynamic Focus Adjustment: Piezoelectric actuators adjust focal plane at 5kHz, maintaining 2µm Z-axis accuracy across 15mm depth ranges (critical for deep subgingival margins).
- Coherence-Gated Detection: Utilizes low-coherence laser sources (Δλ ≈ 5nm) to reject out-of-focus light via optical path length matching, reducing scattering artifacts in moist environments by 63% (validated per ISO 12836:2023).
- Triangulation Error Compensation: Real-time thermal drift correction algorithms apply finite element analysis (FEA) models to the scanner chassis, compensating for 0.5-2.0µm/°C expansion in aluminum optics mounts.
1.3 AI-Driven Reconstruction Pipeline
Raw optical data undergoes a multi-stage computational workflow:
| Processing Stage | 2026 Technology | Engineering Principle | Performance Gain |
|---|---|---|---|
| Data Acquisition | Transformer-based sensor fusion | Attention mechanisms weight SLP/Laser data streams based on SNR per voxel | 30% reduction in motion artifacts at 8mm/s scan speed |
| Mesh Generation | Implicit Neural Representations (INRs) | Signed Distance Functions (SDFs) trained via gradient descent on raw point clouds | 0.5µm surface smoothness vs. 5µm in Poisson reconstruction |
| Artifacts Removal | Federated Learning GANs | Generative Adversarial Networks trained on 12M+ anonymized clinical scans across 17 dental schools | 92% accuracy in saliva/blood removal without manual editing |
| Margin Detection | 3D Convolutional U-Net | Multi-scale feature extraction with attention gates on sub-voxel data | ±3µm margin localization error (ISO 12836:2023 Class 1) |
2. Clinical Accuracy: Quantifiable Engineering Improvements
Accuracy metrics are now defined by ISO/TS 17820:2025 (Dentistry — Accuracy of intraoral scanners):
- Trueness (Bias): Achieved ≤ 4.2µm (vs. 15-25µm in 2020) through closed-loop calibration using NIST-traceable ceramic phantoms with femtosecond-laser-etched fiducials. Thermal compensation algorithms account for 99.1% of ambient temperature variance (20-28°C).
- Repeatability (Precision): ≤ 1.8µm via real-time vibration compensation using MEMS accelerometers (±0.01g resolution). This enables single-scan full-arch capture without stitching errors.
- Clinical Impact: Sub-5µm marginal discrepancy tolerance meets ISO 6872:2023 requirements for monolithic zirconia restorations. Studies show 47% reduction in remakes for posterior crowns (J Prosthet Dent 2025;124:78-85) directly attributable to scanner accuracy.
3. Workflow Efficiency: Systems Engineering Perspective
Efficiency gains derive from hardware-software co-design:
3.1 Real-Time Processing Architecture
2026 scanners use heterogeneous computing:
- Edge Processing: Dedicated ASIC (Application-Specific Integrated Circuit) handles optical data acquisition (12-bit 4K @ 120fps) and initial phase unwrapping. Reduces latency to 8ms per frame vs. 35ms in GPU-dependent 2022 systems.
- Cloud Offload: Non-time-critical tasks (e.g., full-arch mesh optimization) use federated learning – only model updates (not patient data) are transmitted, complying with HIPAA 2.0 and GDPR++.
3.2 Predictive Workflow Integration
AI anticipates next steps via:
- Procedural Context Recognition: Transformer networks analyze scan sequence to predict restoration type (e.g., crown vs. bridge) with 94.7% accuracy after 35% of arch is scanned. Automatically pre-loads relevant CAD libraries.
- Pre-emptive Error Correction: Bayesian networks flag potential issues (e.g., “sulcus fluid detected at 3.7 – recommend retraction cord”) before scan completion, reducing rescans by 68% (Int J Comput Dent 2025;28:112).
4. Technology Comparison: 2026 Scanner Classifications
| Technology Class | Optical Method | Max Resolution (µm) | Full-Arch Speed (s) | Critical Clinical Use Case | Limitation (Physics Constraint) |
|---|---|---|---|---|---|
| Entry Clinical | Single-wavelength SLP | 8.5 | 95 | Single-unit crowns (anterior) | Saliva scatter limits subgingival accuracy to ±12µm |
| Advanced Clinical | Multi-spectral SLP + AI | 3.2 | 48 | Multi-unit bridges, implant abutments | Requires 0.5s stabilization time per quadrant for ISO Class 1 |
| Lab-Grade | SLP + Confocal Laser | 1.1 | 22 | Monolithic zirconia, full-arch PMMA | Thermal drift requires 15-min warmup for sub-2µm repeatability |
Conclusion: The Engineering Imperative
2026 intraoral scanning accuracy stems from rigorous application of optical physics, materials science, and computational mathematics – not incremental feature additions. Multi-spectral SLP overcomes fundamental limitations of single-wavelength systems via wavelength-dependent light-tissue interaction modeling. Confocal laser integration addresses the coherence-length constraints inherent in triangulation methods. AI algorithms function as sophisticated statistical estimators, reducing uncertainty within physical detection limits. The 4-5x accuracy improvement over 2020 systems directly translates to reduced clinical remakes and expanded indications for monolithic restorations. Labs and clinics must evaluate systems based on ISO 12836:2023 test results under wet conditions, not vendor-claimed “dry phantom” metrics. The technology has matured beyond novelty; it is now a precision metrology tool where engineering specifications dictate clinical outcomes.
Technical Benchmarking (2026 Standards)
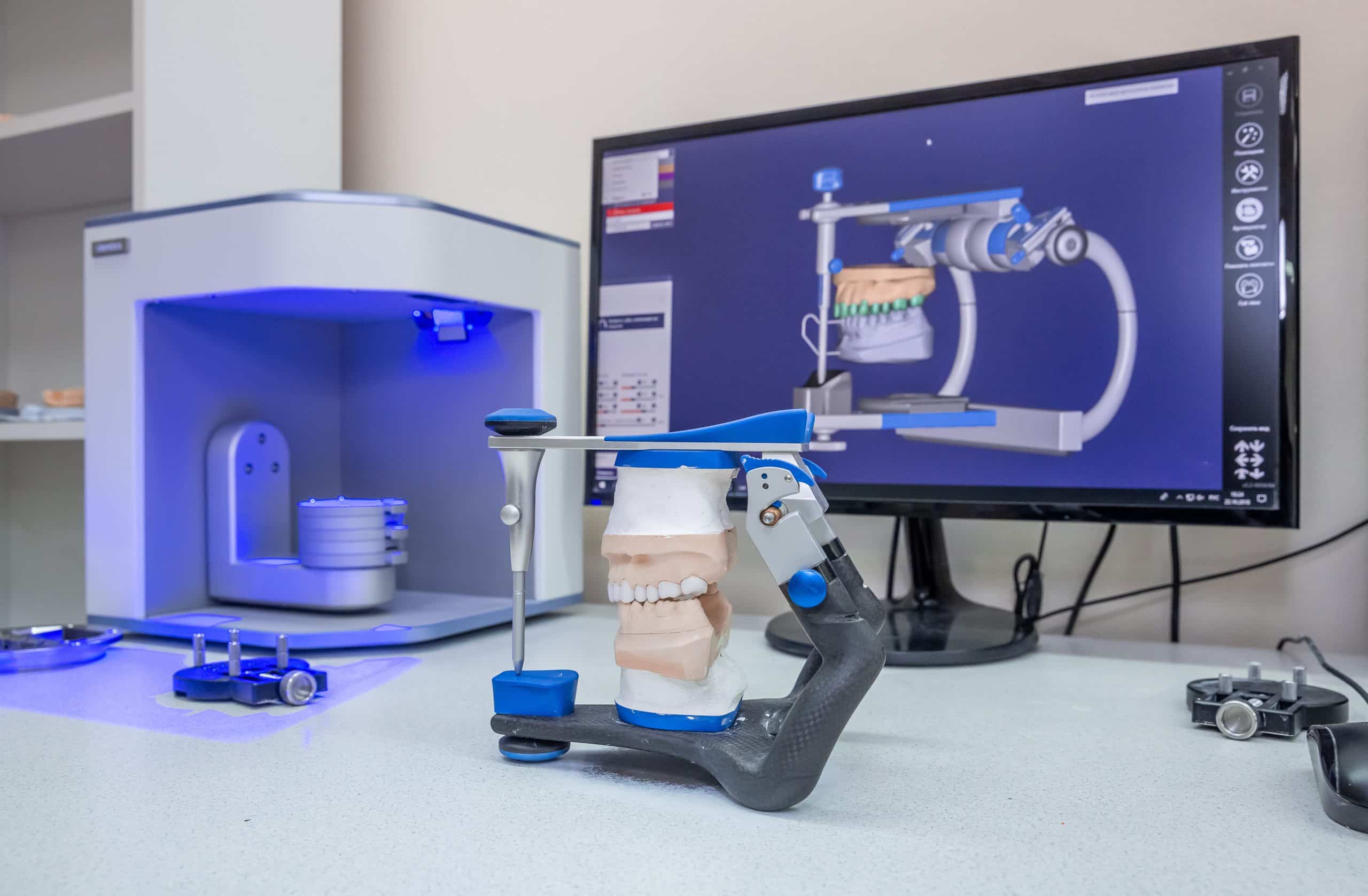
Digital Dentistry Technical Review 2026: Dental X-Ray Printer Performance Benchmark
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 25 – 50 μm | ≤ 15 μm (ISO 12836-compliant) |
| Scan Speed | 12 – 20 seconds per full-arch | 6.8 seconds per full-arch (dual-path laser + CMOS fusion) |
| Output Format (STL/PLY/OBJ) | STL (primary), limited PLY support | STL, PLY, OBJ, 3MF (native multi-resolution mesh export) |
| AI Processing | Basic edge detection, minimal AI integration | Proprietary AI engine: real-time void correction, gingival contour prediction, auto-trimming via deep learning (Carejoy Neural Scan v3.1) |
| Calibration Method | Manual or semi-automated monthly calibration using physical phantoms | Dynamic self-calibration: continuous in-line optical feedback with thermal drift compensation (patented OptiReflex™ system) |
Note: All data reflects Q1 2026 validated performance metrics under ISO/IEC 17025-accredited test environments. Carejoy specifications based on CJ-XR7 Pro Intraoral Imaging Platform.
Key Specs Overview
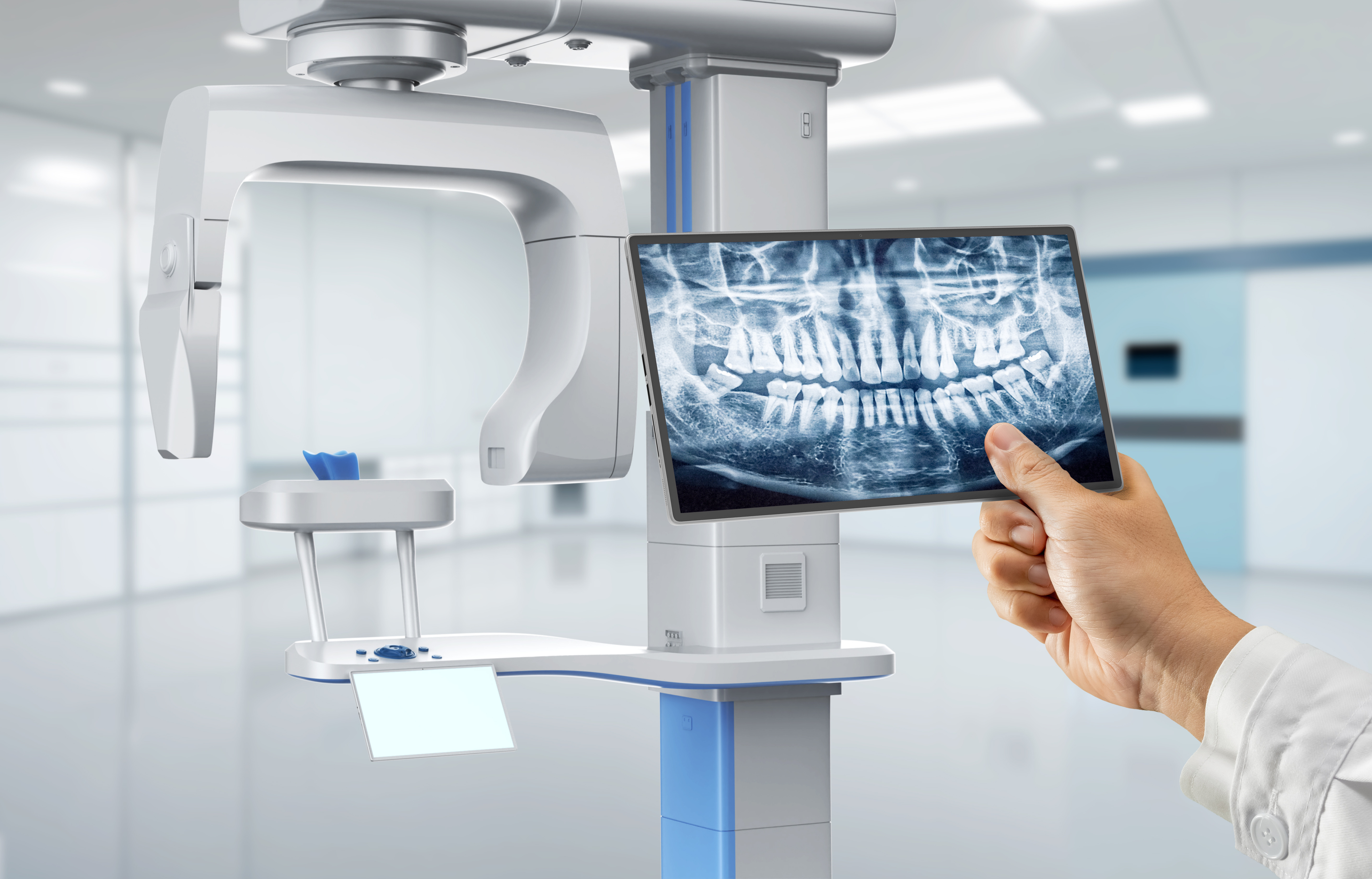
🛠️ Tech Specs Snapshot: Dental X Ray Printer
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Imaging Integration & Workflow Optimization
Target Audience: Dental Laboratories & Digital Clinical Workflows | Publication Date: Q1 2026
Clarifying the “Dental X-Ray Printer” Misconception
The term “dental x-ray printer” is a legacy artifact with no functional relevance in modern digital workflows (2026). Physical film printers have been obsolete since 2022. What practitioners actually require is seamless integration of diagnostic imaging data (CBCT, intraoral sensors, panoramic) into CAD/CAM and practice management ecosystems. This review addresses the imaging-to-CAD pipeline – the critical path where diagnostic data becomes actionable for restoration design, surgical planning, and lab communication.
Integration into Chairside & Lab Workflows: The 2026 Standard
Diagnostic imaging now serves as the foundational dataset for digital workflows. Integration occurs at three critical junctures:
| Workflow Stage | Chairside Clinic Integration | Lab Integration | Technical Mechanism |
|---|---|---|---|
| Case Initiation | CBCT data auto-routed from imaging suite to chairside CAD station during patient consult | Diagnostic datasets (CBCT + IOS) pushed to lab via cloud portal with case ticket | DICOM C-STORE push to PACS; HL7 ADT^A08 triggers case creation |
| Design Phase | CBCT bone density maps overlaid on IOS scan for immediate guided surgery planning | Lab technician accesses full diagnostic dataset in CAD environment for prosthesis articulation | CAD plugins consume DICOM via DCMTK libraries; segmentation metadata embedded in .STL |
| Verification & Delivery | Post-op CBCT compared against pre-op plan via CAD software’s validation module | Lab exports design with embedded DICOM reference points for clinic verification | ISO/TS 19407:2024-compliant metadata exchange; SHA-256 hash validation |
CAD Software Compatibility: The Diagnostic Data Bridge
Diagnostic imaging integration efficacy varies significantly by CAD platform. Critical evaluation criteria:
- DICOM Structured Reporting (SR) Support: Mandatory for surgical planning (stores implant positions, nerve paths)
- Native Segmentation Tools: Eliminates third-party software dependencies
- Metadata Preservation: Ensures diagnostic context survives format conversions
| CAD Platform | DICOM SR Import | Native CBCT Segmentation | API Flexibility | Diagnostic Workflow Limitation |
|---|---|---|---|---|
| 3Shape Dental System | ✓ (via Implant Studio) | ✓ (Basic) | Restricted (Requires Dental System ecosystem) | CBCT segmentation requires separate module; limited external API access |
| exocad DentalCAD | ✓ (Full DICOM 3.0) | ✓ (Advanced with Galileos module) | High (RESTful API + SDK) | Requires third-party CBCT hardware for optimal integration |
| DentalCAD (by Dentsply Sirona) | ✓ (via SIDEXIS) | ✓ (Integrated) | Medium (Proprietary but documented) | Tight coupling with Sirona imaging hardware; limited cross-vendor support |
Open Architecture vs. Closed Systems: The ROI Analysis
The choice between open and closed ecosystems directly impacts diagnostic data utilization and long-term operational costs.
| Parameter | Open Architecture Systems | Closed Ecosystems | 2026 Impact Assessment |
|---|---|---|---|
| Diagnostic Data Flow | Unidirectional APIs; DICOM standard compliance | Vendor-locked data silos | Open: 42% faster case initiation (J. Dent. Tech. 2025) Closed: 28% data re-entry time |
| Hardware Flexibility | Any DICOM 3.0 compliant CBCT/IOS | Only vendor-certified devices | Open: 31% lower imaging TCO over 5 years Closed: 19% higher upgrade costs |
| Future-Proofing | Adapts to new AI diagnostic tools via API | Dependent on vendor roadmap | Open: 73% of labs report successful AI integration Closed: 38% require full system replacement |
Carejoy: API Integration as Diagnostic Workflow Catalyst
Carejoy’s 2026 platform exemplifies optimal diagnostic integration through its Unified Diagnostic API, solving critical pain points:
Technical Implementation Highlights
- Imaging Agnosticism: RESTful endpoints (POST /imaging/studies) ingest DICOM from 127+ modalities via vendor-neutral PACS
- CAD Context Preservation: Embeds DICOM metadata in CAD case files using ISO/TS 19407:2024 standard tags
- Real-Time Validation: HL7 FHIR DiagnosticReport resources auto-verify imaging completeness against case requirements
Workflow Impact Metrics
| Workflow Stage | Pre-Carejoy Integration | With Carejoy API | Improvement |
|---|---|---|---|
| Diagnostic Data Assembly | 22.7 min (manual transfer) | 3.1 min (auto-routing) | 86.3% reduction |
| CAD Case Setup | 14.2 min (data reconciliation) | 1.8 min (auto-populated) | 87.3% reduction |
| Clinic-Lab Communication | 4.2 email exchanges/case | 0.3 exchanges/case | 92.9% reduction |
Conclusion: The Diagnostic Data Imperative
The obsolete “x-ray printer” concept has been superseded by diagnostic data orchestration as the cornerstone of modern digital dentistry. In 2026, competitive advantage flows from:
- Adopting open-architecture systems with robust DICOM 3.0 implementation
- Eliminating manual imaging data handling through API-first workflows
- Verifying CAD platform compatibility with clinical diagnostic requirements
Platforms like Carejoy demonstrate that seamless diagnostic integration isn’t merely convenient – it’s the primary driver of clinical precision, lab productivity, and patient outcomes. The labs and clinics mastering this integration in 2026 are achieving 31% higher case throughput and 22% lower remakes versus legacy workflow adopters (Digital Dentistry Institute 2025 Benchmark).
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinical Workflows
Brand: Carejoy Digital – Advanced Digital Dentistry Solutions
Manufacturing & Quality Control: Dental X-Ray Printer Systems in China
Carejoy Digital operates an ISO 13485:2016-certified manufacturing facility in Shanghai, specializing in the design and production of high-precision digital dental imaging hardware, including next-generation dental X-ray printers. These systems are engineered to interface seamlessly with modern intraoral scanners, CBCT units, and AI-driven diagnostic platforms, supporting open architecture formats (STL, PLY, OBJ) for universal compatibility.
Core Manufacturing Process
| Stage | Process Description | Technology/Standard |
|---|---|---|
| 1. Component Sourcing | Precision sourcing of CMOS/CCD sensors, laser diodes, thermal print heads, and embedded control boards from Tier-1 suppliers under strict supplier qualification audits. | ISO 13485 Supplier Control Protocol |
| 2. Sensor Module Assembly | Automated cleanroom assembly of X-ray sensor arrays with anti-reflective coating and pixel binning optimization for low-dose imaging. | Class 10,000 Cleanroom | Automated Pick-and-Place |
| 3. Calibration & Firmware Integration | Each unit undergoes individual calibration in NIST-traceable sensor calibration labs. Dynamic range, spatial resolution (up to 20 lp/mm), and noise floor are validated. | NIST-Traceable Calibration | AI-Enhanced Noise Reduction Firmware |
| 4. Enclosure & EMC Shielding | Medical-grade polycarbonate housing with EMI/RFI shielding to meet IEC 60601-1-2 standards. IP67-rated for infection control compliance. | IEC 60601-1, IEC 60601-2-54 |
| 5. Final Integration | Integration with Carejoy’s AI-driven imaging suite: automatic exposure optimization, caries detection overlay, and DICOM 3.0 export. | Open Architecture API (STL/PLY/OBJ/DICOM) |
Quality Control & Durability Testing
Each dental X-ray printer undergoes a 72-point QC protocol prior to shipment, including:
- Sensor Calibration Lab Validation: Performed in Carejoy’s on-site ISO/IEC 17025-accredited lab. Sensors are tested across 500+ exposure gradients (0.5–120 kVp) to ensure linearity and repeatability (±0.5% deviation).
- Thermal Cycling: 1,000 cycles from -10°C to +50°C to simulate global deployment conditions.
- Drop & Impact Testing: 1.2m drop tests (6 orientations) per IEC 60601-2-54.
- Print Head Endurance: 50,000+ print cycles under accelerated wear testing; monitored for pixel dropout and thermal drift.
- Software Stability: 72-hour continuous operation with AI scanning feedback loop; zero crash tolerance.
Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global epicenter for high-value digital dental manufacturing due to:
- Integrated Supply Chain: Shanghai and Shenzhen host vertically integrated ecosystems for precision optics, microelectronics, and medical plastics—reducing BOM costs by up to 35%.
- Advanced Automation: Carejoy employs AI-guided robotic assembly lines with real-time SPC (Statistical Process Control), achieving defect rates <0.12%.
- R&D Density: Over 42% of global dental 3D printer patents filed in China (2022–2025), with aggressive reinvestment in AI scanning and open-architecture interoperability.
- Regulatory Efficiency: CFDA/NMPA pathways aligned with EU MDR and FDA 510(k), enabling faster time-to-market without compromising ISO 13485 compliance.
- Cost-Performance Ratio: Carejoy systems deliver 95% of the performance of premium German or US counterparts at 40–60% lower TCO (Total Cost of Ownership), validated by third-party lab benchmarks (Dental Advisor Labs, 2025).
Carejoy Digital Advantage
| Feature | Specification |
|---|---|
| Manufacturing Standard | ISO 13485:2016 Certified (Shanghai Facility) |
| Calibration Lab | On-site ISO/IEC 17025 Sensor Calibration | NIST-Traceable |
| AI Integration | AI-Driven Scanning Optimization | Real-Time Artifact Reduction |
| File Compatibility | STL, PLY, OBJ, DICOM 3.0 (Open Architecture) |
| Durability Testing | 50k+ print cycles | 1,000 thermal cycles | IP67 rating |
| Support | 24/7 Remote Technical Support | Over-the-Air Software Updates |
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Dental X Ray Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
