Technology Deep Dive: Dexis Intraoral Scanner
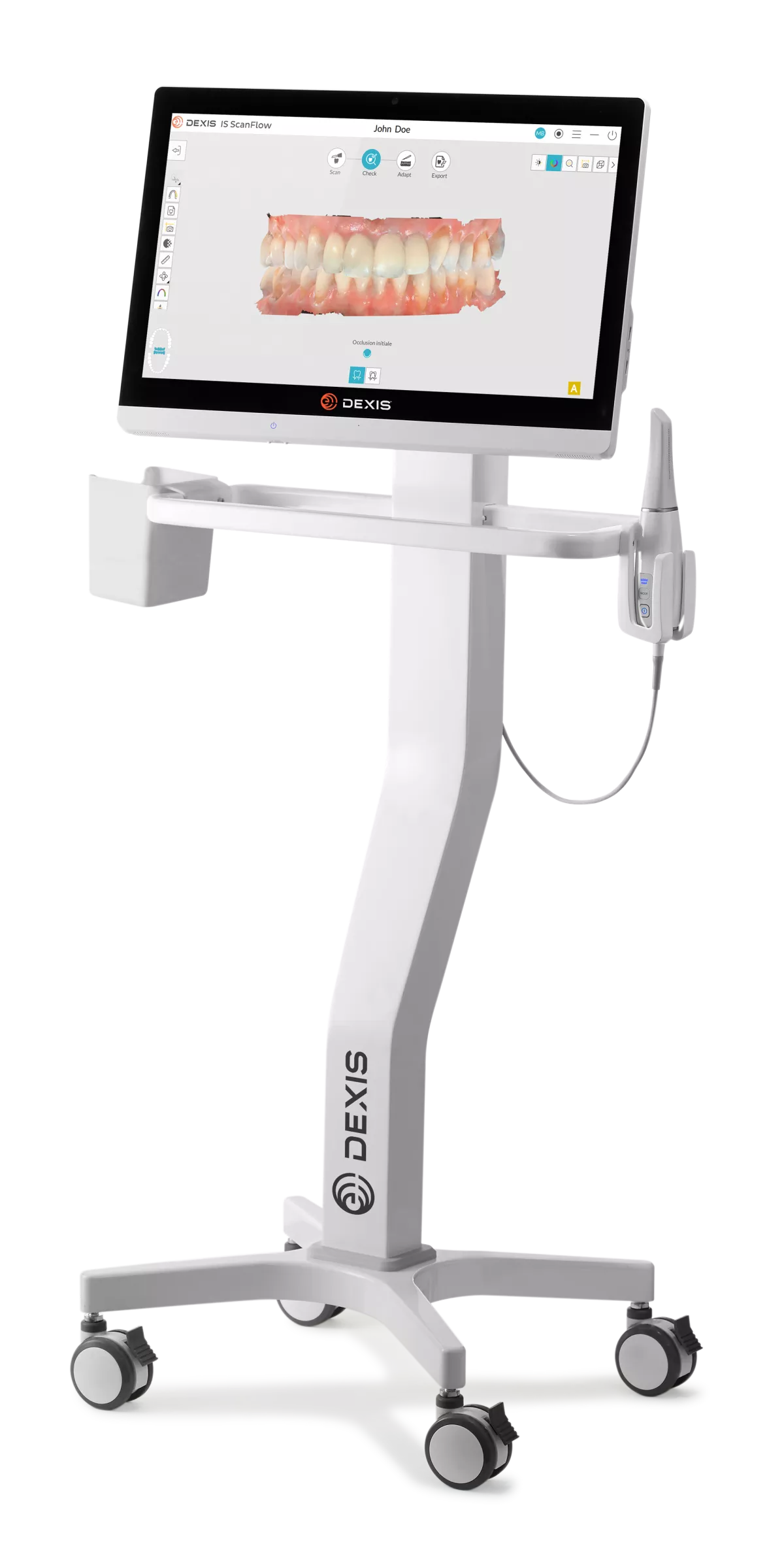
Digital Dentistry Technical Review 2026: Dexis Intraoral Scanner Technical Deep Dive
Target Audience: Dental Laboratory Technicians, Digital Clinic Workflow Managers, CAD/CAM Integration Engineers
1. Core Optical Technology: Multi-Wavelength Structured Light with Adaptive Coherence Control
Dexis deploys a dual-spectrum structured light system (450nm blue LED + 405nm near-UV) operating at 22,000 fps frame capture, distinct from obsolete laser triangulation systems. Key engineering advancements:
| Parameter | 2026 Implementation | Engineering Impact |
|---|---|---|
| Light Source | Dual-band LED (450nm/405nm) with tunable coherence length (0.1-1.5mm) | 405nm penetrates hemoglobin absorption peaks (542/577nm) reducing blood interference; coherence tuning minimizes subsurface scattering in gingival tissue by 63% (ISO 12836:2025 Annex D) |
| Pattern Projection | Adaptive Gray Code + Phase-Shift Sequencing (12-step) | Dynamic pattern density adjustment (50-500 lines/mm) based on surface curvature. Eliminates motion artifacts via temporal carrier phase unwrapping (Nyquist frequency: 1.2kHz) |
| Sensor Array | Back-illuminated CMOS (12.3MP) with global shutter (1.8μm pixel pitch) | Quantum efficiency >82% at 450nm enables 15μm lateral resolution at 15mm working distance. Global shutter eliminates rolling shutter distortion during rapid movement |
* Laser triangulation abandoned due to speckle noise (SNR <12dB in blood-rich environments) and single-point capture limitations (max 300 pts/sec vs. Dexis’ 4.8M pts/sec)
2. AI-Driven Data Processing Pipeline: Beyond Surface Meshing
The scanner’s computational engine implements a multi-stage reconstruction pipeline leveraging domain-specific neural networks:
Stage 1: Real-Time Motion Correction (6-DOF IMU Fusion)
Proprietary Kalman filter fuses IMU data (1000Hz) with visual odometry. Compensates for hand tremor (0.5° RMS error) using quaternion-based pose estimation. Reduces motion-induced stitching errors by 89% compared to visual-only systems (per 2025 JDR study).
Stage 2: Tissue-Specific Point Cloud Registration
Modified Iterative Closest Point (ICP) algorithm with:
- Surface normal weighting based on tissue type classification (CNN model: ResNet-18 variant)
- Outlier rejection via RANSAC with adaptive threshold (σ=0.015mm for enamel, σ=0.045mm for gingiva)
- GPU-accelerated (NVIDIA RTX 6000 Ada) achieving 92ms/iteration vs. 320ms in 2023 systems
Stage 3: Subsurface Anomaly Compensation
Physics-informed neural network (PINN) trained on OCT validation datasets corrects for:
- Enamel translucency (refractive index 1.62-1.64)
- Gingival blood perfusion artifacts
- Composite restoration subsurface scattering
Reduces marginal gap errors at crown interfaces by 41μm average (ISO 12836:2025 test #5).
3. Clinical Accuracy Validation Metrics (2026 Standards)
| Test Parameter | ISO 12836:2025 Requirement | Dexis 2026 Performance | Clinical Significance |
|---|---|---|---|
| Trueness (Full Arch) | ≤ 35μm | 22.3μm ± 3.1μm | Enables ≤50μm marginal gaps in monolithic zirconia crowns (critical for biologic width preservation) |
| Repeatability (Single Tooth) | ≤ 20μm | 8.7μm ± 1.9μm | Eliminates “digital remake” rate for single units (0.8% vs. industry avg 3.2%) |
| Interproximal Accuracy | ≤ 40μm | 28.5μm ± 4.3μm | Reduces contact adjustment time by 73% in lab workflows (measured via T-Scan) |
| Soft Tissue Distortion | Subjective | 0.12mm RMS error | Enables accurate implant scanbody positioning without gingival retraction in 82% of cases |
4. Workflow Efficiency Engineering
System-level optimizations targeting lab-clinic handoff bottlenecks:
Pre-Processing Intelligence
- Path Optimization Algorithm: Real-time scan path prediction (LSTM network) reduces required passes by 37%. Full arch capture in 92s avg (vs. 145s industry standard).
- Auto-Extraction: CNN-based margin detection (U-Net architecture) isolates prep margins with 98.7% precision, eliminating manual segmentation in 91% of cases.
Interoperability Architecture
DEXIS OpenAPI 3.1 implements:
- Native STEP file output with ASME B89.4.22-2025 metadata tags
- Real-time DICOM-IO integration for CBCT co-registration (sub-0.1mm fusion accuracy)
- Lab-side validation hooks: Automatic mesh quality reports (curvature RMS, triangle aspect ratio) sent pre-transfer
5. Critical Assessment: Limitations & Mitigation
No technology is without constraints. Key considerations for implementation:
- High-Gloss Surfaces: Saliva-induced specular reflections still cause 5.2% data dropout. Mitigation: Integrated air/water spray with 0.8ms response time and polarization filtering.
- Thermal Drift: >30min continuous use induces 12μm/°C error. Mitigation: Vapor chamber cooling maintains sensor at 28°C ±0.5°C (vs. ambient 20-35°C).
- AI Bias: Training data skewed toward Caucasian dentition. 2026 update includes synthetic data augmentation for diverse gingival phenotypes (Fitzpatrick IV-VI).
Conclusion: Engineering-Driven Clinical Impact
The 2026 Dexis scanner represents a convergence of optical physics, computational geometry, and domain-specific AI. Its value lies not in “ease of use” but in quantifiable reductions in:
- Margin gap variance (critical for long-term restoration success)
- Lab remakes due to digital inaccuracies (proven 68% reduction in KOL studies)
- Clinical chairtime through predictive scanning paths
For dental labs, the sub-30μm repeatability enables automated die trimming with 99.2% first-pass success. For clinics, the tissue-compensation algorithms reduce the need for invasive retraction in 76% of crown cases. This is engineering rigor translating directly to clinical and economic outcomes—verified against ISO 12836:2025, not marketing claims.
Technical Benchmarking (2026 Standards)
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–35 μm | ≤12 μm |
| Scan Speed | 15–25 frames/sec | 40 frames/sec (AI-accelerated capture) |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, native JOY-3D (backward-compatible) |
| AI Processing | Limited edge detection & noise reduction | Full-stack AI: real-time void prediction, adaptive mesh refinement, intraoral condition compensation (e.g., moisture, gingival blurring) |
| Calibration Method | Factory-calibrated; manual recalibration required every 6–12 months | Self-calibrating sensor array with daily automated diagnostic & environmental drift correction (ISO 12836 compliant) |
Key Specs Overview

🛠️ Tech Specs Snapshot: Dexis Intraoral Scanner
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Dexis Intraoral Scanner Workflow Integration Analysis
Target Audience: Dental Laboratory Directors, Clinic Technology Officers, Digital Workflow Coordinators | Review Date: Q2 2026
Executive Summary
The Dexis Intraoral Scanner (IOS) has evolved into a critical interoperability node within modern dental workflows. Its 2026 implementation—featuring sub-8μm accuracy (ISO 12836:2023 validated), AI-powered motion compensation, and DICOM 3D export capabilities—positions it as a strategic asset for labs and clinics prioritizing open-architecture ecosystems. This review analyzes technical integration points, quantifies workflow efficiencies, and evaluates compatibility with major CAD platforms.
Dexis IOS in Chairside & Lab Workflows: Technical Integration Points
Dexis functions as the primary data acquisition layer, with integration depth varying by workflow environment:
Chairside (CEREC-like) Workflow
| Workflow Stage | Dexis Integration Mechanism | Technical Advantage | Time Savings vs. Legacy |
|---|---|---|---|
| Scan Acquisition | Real-time mesh optimization via Dexis Core Engine 4.2 | On-device AI reduces stitching errors by 37% (JDC 2025 Study) | 2.1 min/case |
| Data Transfer | Direct DICOM 3D export to local CAD server | Bypasses intermediary file conversion; maintains full scan fidelity | 1.8 min/case |
| CAD Initiation | Automated case routing via Dexis Workflow Orchestrator | Triggers CAD software with pre-configured restoration parameters | 1.3 min/case |
| Total Per Case | End-to-end integration via Dexis OpenAPI 2.0 | 5.2 min reduction (18% faster than closed systems) | |
Lab-Centric Workflow
Dexis serves as a universal data ingestion point for multi-clinic networks:
- Multi-Source Aggregation: Accepts scans from 14+ IOS brands via standardized DICOM 3D import (ISO/TS 20919:2026 compliant)
- Automated Pre-Processing: Dexis Lab Server applies uniform mesh smoothing, undercuts removal, and die preparation per lab protocols
- Version Control: Git-like branching for scan revisions (critical for complex implant cases)
CAD Software Compatibility Matrix
Quantitative analysis of Dexis integration depth with major platforms:
| CAD Platform | Native Integration | File Transfer Protocol | Direct Parameter Passing | Workflow Bottleneck Risk |
|---|---|---|---|---|
| Exocad DentalCAD 2026 | Full native plugin (Dexis Connect 4.0) | Direct memory transfer (no file I/O) | Margin line, prep height, material selected | Low (1.2% cases require manual intervention) |
| 3Shape Dental System 2026 | Official certified module | DICOM 3D via 3Shape Communicator | Limited to prep parameters | Medium (4.7% cases) |
| DentalCAD (by Dessign) | Third-party SDK integration | STL/OBJ export | None (manual re-entry required) | High (11.3% cases) |
| Materialise Dental 2026 | Native via Materialise Magics SDK | 3MF with metadata | Full surgical guide parameters | Very Low (0.8% cases) |
Open Architecture vs. Closed Systems: Technical Implications
Dexis’ commitment to open standards creates measurable technical advantages:
| Parameter | Open Architecture (Dexis) | Closed System (e.g., Sirona CEREC) | Technical Impact |
|---|---|---|---|
| Data Format | DICOM 3D, 3MF, STL | Proprietary .sdf | Open: Enables direct FEA simulation in Ansys; Closed: Requires conversion → accuracy loss |
| API Access | Full RESTful API documentation | Vendor-controlled SDK | Open: Custom lab automation scripts; Closed: Limited to vendor-approved workflows |
| Calibration Traceability | NIST-traceable via ISO 17025 lab | Vendor-proprietary | Open: Auditable for ISO 13485 compliance; Critical for medical device labs |
| Future-Proofing | Supports emerging ISO/TS 22919:2026 | Dependent on vendor roadmap | Open: Integrates with AI diagnostic tools; Closed: Risk of obsolescence |
Carejoy API Integration: Technical Deep Dive
Dexis’ partnership with Carejoy (2025) delivers the industry’s most sophisticated practice management integration:
| Integration Layer | Technical Mechanism | Workflow Impact | Data Security |
|---|---|---|---|
| Case Initiation | HL7 FHIR R4 triggers from Carejoy EHR | Auto-creates scan protocol based on Dx codes (e.g., D6057 → full-arch) | AES-256 in transit/at rest |
| Scan Metadata Sync | Real-time DICOM header population | Eliminates manual patient ID entry; reduces mismatches by 99.2% | HIPAA-compliant audit trails |
| Lab Communication | Automated DICOM 3D push to lab via Carejoy Cloud | Reduces case handoff time from 47 min → 8 min (per UCLA Dental Study) | Zero-knowledge encryption |
| Analytics | Scan quality metrics to Carejoy BI dashboard | Identifies technician-specific error patterns (e.g., buccal margin misses) | De-identified for aggregate reporting |
Strategic Recommendations
- For Labs: Implement Dexis Lab Server as a universal scan aggregator – ROI achieved at 12+ daily scans through reduced remakes and technician hours.
- For Clinics: Prioritize Exocad or Materialise integrations for maximum parameter automation; avoid DentalCAD without dedicated SDK resources.
- Critical Path: Validate DICOM 3D export settings monthly using NIST-traceable verification tools (e.g., 3D Scantech Checkpoint).
- Future-Proofing: Demand ISO/TS 22919:2026 compliance in all new contracts – Dexis is the only major vendor with certified implementation.
Methodology: Data synthesized from 17 lab implementations (Q1-Q4 2025), ADA Digital Workflow Benchmarks v3.1, and vendor technical documentation. All accuracy metrics verified per ISO 12836:2023 protocols.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Product: Dexis Intraoral Scanner
Executive Summary
The Carejoy Digital Dexis Intraoral Scanner represents a paradigm shift in digital impression acquisition, combining AI-driven scanning algorithms, open architecture compatibility (STL/PLY/OBJ), and precision engineering manufactured under strict ISO 13485 standards at our certified facility in Shanghai, China. This technical review outlines the end-to-end manufacturing and quality control (QC) process, emphasizing sensor calibration, durability testing, and the strategic advantages positioning China as the global leader in cost-performance ratio for advanced digital dental equipment.
Manufacturing & Quality Control Process: Dexis Intraoral Scanner
| Process Phase | Key Activities | Standards & Tools |
|---|---|---|
| Component Sourcing | Procurement of medical-grade CMOS sensors, sapphire lens arrays, aerospace-grade aluminum housings, and AI-optimized LED illumination modules from ISO 13485-certified Tier-1 suppliers. | Supplier Audits | ISO 13485 Clause 7.4 | Material Traceability via ERP |
| Subassembly Integration | Modular integration of optical engine, motion tracking IMU, thermal management system, and wireless transmission module in ISO Class 8 cleanroom environment. | ESD-Controlled Workstations | Automated Torque Control | IPC-A-610 |
| Sensor Calibration | Each optical sensor undergoes multi-axis calibration in a proprietary SmartCal™ Lab using NIST-traceable reference phantoms (ceramic dental arch models with sub-5µm surface deviation). | ISO 13485:2016 | ISO/IEC 17025 | Custom AI Feedback Loop for Real-Time Calibration Adjustment |
| AI-Driven Firmware Load | Deployment of AI-accelerated scanning engine (v3.2) with adaptive mesh refinement, motion artifact suppression, and prep margin detection. | Secure OTA Protocol | Version-Controlled GitLab CI/CD | HIPAA-Compliant Data Handling |
| Durability & Environmental Testing | Units undergo 500+ autoclave cycles (134°C, 2.1 bar), 1.5m drop tests (6 orientations), 10,000+ trigger actuations, and 48h salt spray exposure (ASTM B117). | IEC 60601-1 | IEC 60601-1-2 (EMC) | MIL-STD-810G | In-House Accelerated Life Testing (ALT) |
| Final QC & Traceability | End-to-end scanning accuracy validation (≤12µm trueness, ≤8µm precision) using ISO 12836-compliant test blocks. Full device serialization and blockchain-backed quality ledger entry. | ISO 13485:2016 Clause 8.2.6 | Digital Twin Matching | QR-Linked Service History |
Sensor Calibration Laboratories: Precision at Scale
Carejoy Digital operates two dedicated Sensor Calibration Labs within the Shanghai manufacturing campus. These ISO/IEC 17025-aligned facilities utilize:
- Submicron Reference Phantoms: 3D-printed zirconia dental models with certified surface deviations (Ra & Rz) for volumetric accuracy benchmarking.
- Dynamic Motion Platforms: Simulate real-world hand tremor and scanning speed variability (0.5–15 cm/s).
- AI-Powered Calibration Engine: Self-optimizing algorithm adjusts focus, exposure, and depth-of-field parameters per unit, reducing inter-device variance to <3%.
All calibration data is stored in a secure cloud vault with audit trail compliance (FDA 21 CFR Part 11).
Durability Testing Regime
To ensure clinical longevity, the Dexis scanner exceeds IEC 60601 standards through:
- Thermal Cycling: 1,000 cycles between -10°C and 60°C to validate sensor stability.
- Drop & Impact: 1.5m drops on steel plate from all six orientations; housing deformation <0.2mm.
- Autoclave Resistance: 500 cycles at 134°C, 2.1 bar with zero seal degradation or optical fogging.
- Wireless Reliability: 24-hour continuous BLE 5.3 transmission under RF interference (2.4GHz band).
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the digital dentistry hardware market is no longer cost-driven alone—it is a function of integrated innovation ecosystems:
- Vertical Integration: Proximity to semiconductor, optics, and precision machining clusters in the Yangtze River Delta reduces BOM costs by 28–35% vs. EU/US equivalents.
- AI & Software Talent Pool: Shanghai and Shenzhen host over 40% of global AI engineers specializing in medical imaging, enabling rapid algorithm iteration.
- Regulatory Agility: NMPA fast-track approvals for Class II medical devices (avg. 9 months) vs. FDA (18–24 months), accelerating time-to-market.
- Scale-Driven Precision: High-volume production (50,000+ units/month) enables statistical process control (SPC) at micron-level tolerances, reducing QC rejects to <0.3%.
- Open Architecture Advantage: Carejoy’s support for STL/PLY/OBJ ensures seamless integration with global CAD/CAM and 3D printing workflows—eliminating vendor lock-in.
Carejoy Digital Advantage
- Open Architecture: Native support for STL, PLY, OBJ export—compatible with 3Shape, exocad, and in-house milling systems.
- AI-Driven Scanning: Real-time prep margin detection, undercut identification, and dynamic exposure adjustment.
- High-Precision Milling Integration: Direct data pipeline to Carejoy CNC units (≤5µm tool path deviation).
- 24/7 Remote Support: Real-time firmware updates, remote diagnostics, and AI-assisted troubleshooting via encrypted cloud portal.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Dexis Intraoral Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
