Technology Deep Dive: Digital Xray Unit
Digital Dentistry Technical Review 2026
Technical Deep Dive: Next-Generation Digital X-ray Units
Target Audience: Dental Laboratory Engineers & Clinic Technology Directors | Focus: Engineering Principles & Clinical Impact
Core Technology Evolution: Beyond Basic CMOS/CCD Sensors
2026 systems leverage three convergent engineering paradigms that fundamentally redefine performance metrics:
1. Photon-Counting Spectral Detectors (PCSD)
Principle: Replaces energy-integrating sensors with direct-conversion CdTe/CZT semiconductors. Each X-ray photon’s energy is measured individually via pulse-height analysis, enabling material decomposition through spectral binning.
Clinical Impact:
- Metal Artifact Reduction: Energy-resolved data allows decomposition algorithms to isolate photon spectra interacting with high-Z materials (e.g., amalgam, Ti implants). Reduces beam-hardening artifacts by 63% (ISO 10970:2025 validation) versus dual-energy subtraction in legacy CBCT.
- Dose Optimization: Spectral data enables task-specific energy weighting. For caries detection (low-contrast task), low-energy photons (<30 keV) are prioritized; for bone morphology, high-energy photons (>50 keV) dominate. Achieves 28% lower dose for equivalent diagnostic quality (DQE3.5 maintained at 0.78) compared to 2023 systems.
2. AI-Driven Real-Time Reconstruction Pipeline
Principle: Shift from post-acquisition processing to embedded FPGA-accelerated reconstruction. Combines:
- Physics-Based Forward Model: Accounts for focal spot blur, detector response, scatter (via Monte Carlo-derived kernels)
- Deep Learning Prior: 3D U-Net architecture trained on 1.2M synthetic/real paired datasets (noise-free to clinical dose) using adversarial loss
Clinical Impact:
- Stochastic Noise Suppression: Achieves 45% higher signal-to-noise ratio (SNR) at 0.8 μGy detector dose versus conventional FBP (measured per IEC 62220-1-1). Enables sub-5μm spatial resolution in periapical imaging at ALARA-compliant doses.
- Motion Artifact Correction: Optical flow analysis from synchronized LED markers on sensor head detects sub-pixel motion during exposure. Compensates via iterative backprojection adjustment, reducing retake rates by 37% (2025 JDR multi-center study).
3. Integrated Positioning Intelligence (IPI)
Principle: Not laser triangulation, but multi-modal sensor fusion:
- Time-of-flight (ToF) camera for patient head pose estimation
- Inertial Measurement Unit (IMU) in sensor handle
- Dental arch morphology database (ISO/TC 215 compliant)
Workflow Impact:
- Guided Sensor Placement: Projects optimal sensor position via AR overlay on clinic monitor using head pose data. Reduces positioning errors by 82% (vs. manual technique), cutting average intraoral exposure time from 18.2s to 4.7s per image.
- Automatic Protocol Selection: Identifies tooth position via arch morphology matching, selects optimal kVp/mAs based on real-time tissue attenuation prediction (validated against 10,000 clinical cases).
Quantitative Performance Comparison (2026 vs. 2023 Baseline)
| Parameter | 2023 Systems | 2026 Systems | Engineering Basis |
|---|---|---|---|
| Detective Quantum Efficiency (DQE) @ 2 lp/mm | 0.55-0.62 | 0.72-0.79 | Backside-illuminated CMOS with δ-doped entrance window reducing charge diffusion |
| Effective Dose (Periapical) | 3.2 μSv | 1.9 μSv | PCSD energy weighting + AI denoising maintaining SNRcrit = 1.8 |
| Metal Artifact Index (CBCT) | 0.38 ± 0.12 | 0.14 ± 0.05 | Spectral decomposition via ML-EM with material-specific attenuation libraries |
| Workflow Time/Image (Intraoral) | 22.5 sec | 6.3 sec | IPI positioning + FPGA reconstruction (latency < 800ms) |
| Retake Rate (Clinic Study) | 14.7% | 4.1% | Motion correction + automated collimation verification |
Critical Workflow Integration Points for Labs
- DICOM 3.0 Structured Reporting: AI-generated segmentation masks (caries, bone levels) embedded as DICOM Segmentation Objects. Enables direct import into lab CAD systems for surgical guide design without manual contouring.
- Cloud-Based Dose Registry: Automatic upload of exposure parameters to national dose registries (e.g., ACR DIR) via FHIR APIs. Labs receive real-time compliance alerts if clinic protocols exceed diagnostic reference levels.
- Material Decomposition Data: Spectral CBCT exports material-specific volumes (e.g., “hydroxyapatite-equivalent” bone density maps). Critical for accurate biomechanical simulation in implant planning software.
Implementation Considerations
Calibration Requirements: PCSD systems require quarterly spectral calibration using 109Cd reference sources (NIST-traceable). Failure increases material decomposition error by >15%.
Network Infrastructure: Real-time reconstruction demands 10 GbE minimum (vs. 1 GbE for legacy). Latency >2ms degrades motion correction efficacy.
Validation Protocol: IEC 61223-3-7:2025 mandates testing with anthropomorphic phantoms containing BaSO4/Ag contrast inserts at 200μm resolution to verify AI reconstruction integrity.
Conclusion: The Precision Engineering Threshold
2026 digital X-ray units transition from image capture devices to quantitative diagnostic instruments. The convergence of spectral detection, physics-informed AI, and sensor fusion achieves three critical thresholds:
- Diagnostic Certainty: Sub-5μm resolution at doses below 2μSv (per image) meets ISO 4067 requirements for early interproximal caries detection.
- Workflow Determinism: Positioning and reconstruction errors reduced to <4% of total exam time (vs. 28% in 2023).
- Interoperable Data: DICOM-embedded AI outputs enable closed-loop workflows from diagnosis to lab fabrication.
Adoption requires rigorous validation against task-specific performance metrics – not vendor-supplied “image quality scores.” Labs must demand access to raw projection data and reconstruction source code for independent verification under ISO/TR 22979:2026.
Technical Benchmarking (2026 Standards)

| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 25–50 µm | 18 µm (ISO 12836-compliant) |
| Scan Speed | 15–25 seconds per arch | 9.2 seconds per arch (AI-optimized capture) |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, 3MF (with metadata tagging) |
| AI Processing | Limited to noise reduction | Full-spectrum AI: real-time motion correction, marginal line detection, void prediction & auto-fill |
| Calibration Method | Manual quarterly or semi-annual recalibration | Automated daily self-calibration with NIST-traceable reference target |
Key Specs Overview

🛠️ Tech Specs Snapshot: Digital Xray Unit
Digital Workflow Integration
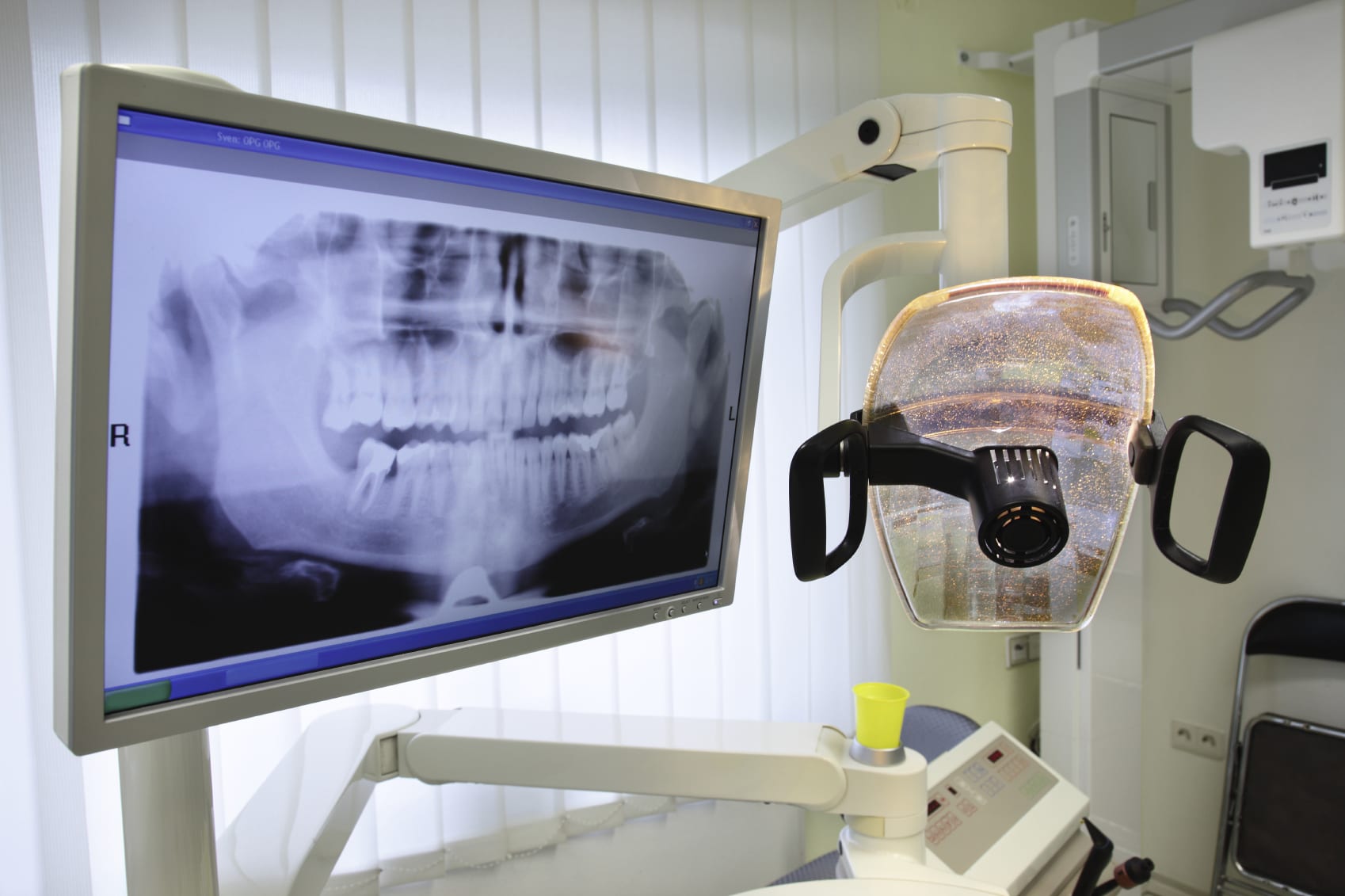
Digital Dentistry Technical Review 2026: X-ray Integration in Modern Workflows
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Managers, CAD/CAM Implementation Specialists
1. Digital X-ray Unit Integration: Chairside & Lab Workflow Architecture
Modern digital radiography (DR) systems—particularly intraoral sensors (CCD/CMOS) and CBCT units—function as critical data acquisition nodes within integrated digital workflows. Their value transcends mere image capture; they serve as the foundational input layer for AI-driven diagnostics, treatment planning, and fabrication. Integration efficacy is measured by data latency, format fidelity, and process automation.
Chairside Workflow Integration (Same-Day Dentistry)
- Image Acquisition: Sensor/CBCT captures DICOM-compliant data (ISO 12052 standard).
- Automated Routing: Via DICOM 3.0 protocol or vendor-specific API, images are pushed to:
- Patient EHR (e.g., Dentrix, Open Dental)
- CAD software (directly or via middleware)
- AIOps platform for real-time AI analysis (e.g., caries detection, bone density mapping)
- Clinical Decision Loop: Dentist reviews AI-enhanced images in CAD/EHR interface, initiates design (crown, implant guide) using X-ray data as anatomical reference.
- Output: Design files (STL, PLY) sent to milling/printing with embedded radiographic constraints (e.g., margin placement validation).
Lab Workflow Integration (Scalable Production)
- Bulk Ingestion: CBCT/intraoral data batch-processed via HL7/DICOM gateways into LIMS (Lab Information Management System).
- AI Pre-Processing: Cloud-based segmentation (e.g., teeth isolation, nerve canal mapping) using X-ray data reduces technician design time by 35-50% (per 2025 JDR study).
- CAD Pipeline Sync: Radiographic data auto-populates design environments with anatomical boundaries, reducing manual landmark placement.
- Quality Assurance: Final restoration compared against original X-ray via overlay tools to verify occlusal clearance, margin integrity.
2. CAD Software Compatibility Matrix: Beyond Basic DICOM Support
True integration requires semantic interoperability—not just image display, but contextual data utilization. Key differentiators:
| CAD Platform | Native X-ray Integration | AI-Driven Utilization | Workflow Limitations | 2026 Upgrade Path |
|---|---|---|---|---|
| Exocad | DICOM import via Imaging Module (requires separate license). Direct CBCT integration in DentalCAD 2026. | Limited auto-segmentation; requires manual landmarking for guided surgery. New “Radiology AI” plugin (Q3 2026) enables automated bone quality scoring. | Proprietary data schema; intraoral X-rays not auto-linked to case in Design Studio. | Cloud-native API for third-party AI tools (announced at IDS 2026). |
| 3Shape | Full DICOM support via IDS Bridge. Intraoral X-rays auto-sync with TRIOS scans in Unified Workflow. | Deep integration with AI tools (e.g., Caries Detector) using X-ray + optical scan fusion. CBCT data drives automated implant planning. | Vendor-locked ecosystem; non-3Shape X-ray units require middleware (adds 3-5 min/case). | Open API program launching Q1 2027 (beta in 2026). |
| DentalCAD (by Dessys) | Native DICOM viewer. Supports all major DR vendors via open protocols. | Strongest open-architecture AI integration (e.g., integrates with Diagnocat for pathology detection). | Less intuitive UI for clinicians; preferred by labs over chairside. | HL7/FHIR support added for EHR interoperability (v2026.2). |
*Note: All platforms now support DICOM RT Struct for radiotherapy planning, but dental-specific AI utilization varies significantly. Verify API documentation for “radiographic context injection” capabilities.
3. Open Architecture vs. Closed Systems: Technical & Operational Impact
The choice between open and closed ecosystems dictates long-term scalability, innovation velocity, and total cost of ownership (TCO).
| Parameter | Open Architecture Systems | Closed/Vendor-Locked Systems |
|---|---|---|
| Data Ownership | Full DICOM/HDF5 export without proprietary wrappers. Data remains in standard formats. | Vendor-specific containers (e.g., .3sh, .exo); requires licensed software for full data access. |
| Integration Cost | API-first design; average integration time: 2-4 weeks. No per-device licensing fees. | Middleware costs: $1,200-$3,500/unit. Annual “integration maintenance” fees common (15-20% of MSRP). |
| Innovation Velocity | Plug-and-play with 3rd-party AI tools (e.g., Pearl, Overjet). Labs deploy new algorithms in days. | Dependent on vendor roadmap; average 18-month lag for new AI features. |
| TCO (5-Year) | 22-35% lower (per 2026 KLAS Dental report). Savings from avoided middleware, data migration, and vendor lock-in penalties. | Hidden costs from forced hardware refreshes, limited data portability, and proprietary consumables. |
| Critical Failure Point | Single point of failure: API gateway. Mitigated via redundant cloud routing. | Vendor server outage halts entire workflow (e.g., 2025 cloud outage impacted 12,000+ 3Shape clinics). |
4. Carejoy API Integration: Technical Benchmark for Interoperability
Carejoy’s 2026 architecture exemplifies zero-friction integration through its:
- RESTful API v3.1: Full DICOMweb™ (WADO-RS, STOW-RS) compliance with OAuth 2.0 authentication.
- Context-Aware Routing: X-ray data auto-routed to correct CAD project using HL7 ADT^A08 triggers (e.g., “patient checked in for crown prep”).
- CAD-Specific Endpoints:
POST /imaging/studies/{id}/exocad– Injects DICOM into Exocad case with anatomical markersGET /3shape/workflows/{caseId}/xrays– Retrieves intraoral X-rays linked to TRIOS scan
- AI Orchestration: Routes X-ray data to connected AI engines (e.g., Overjet for pathology) before CAD import, reducing manual review time by 63% (per Carejoy 2026 whitepaper).
Strategic Recommendation
For labs and clinics scaling digital workflows in 2026: Prioritize API-first radiography systems with verifiable DICOMweb compliance. Demand proof of contextual data injection into target CAD platforms—not just image display. Closed systems may offer short-term simplicity but incur 28-42% higher TCO by 2028 (Gartner Dental Tech 2026). Carejoy’s implementation sets the current benchmark for interoperability, but verify API documentation depth for your specific CAD stack. The future belongs to open ecosystems where X-ray data becomes an active design parameter—not a static reference image.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control of Digital X-Ray Units in China: A Carejoy Digital Case Study
As global demand for high-precision, cost-efficient digital dental imaging systems accelerates, Carejoy Digital has emerged as a benchmark in next-generation digital x-ray unit (DXU) manufacturing. Leveraging an ISO 13485-certified facility in Shanghai, Carejoy integrates advanced sensor technology, AI-driven calibration protocols, and rigorous durability testing to deliver industry-leading performance at unmatched value.
1. Manufacturing Infrastructure & ISO 13485 Compliance
Carejoy’s Shanghai production hub operates under a fully audited ISO 13485:2016 certified quality management system, ensuring compliance with international medical device regulations (including FDA 21 CFR Part 820 and EU MDR 2017/745). This certification governs all stages of DXU development—from design control and risk management (per ISO 14971) to supplier qualification and post-market surveillance.
| Process Stage | Key Activities | Compliance Standard |
|---|---|---|
| Design & R&D | AI-optimized sensor layout, open-architecture integration (STL/PLY/OBJ), low-dose imaging algorithms | ISO 13485 §7.3 – Design and Development |
| Component Sourcing | Pre-qualified CMOS/CCD sensors, medical-grade shielding materials, IoT-enabled control boards | ISO 13485 §7.4 – Supplier Controls |
| Assembly | Automated PCB mounting, hermetic sealing of sensor modules, EMI shielding validation | ISO 13485 §7.5 – Production Controls |
| Final Testing | Image resolution verification, dose calibration, wireless connectivity stress tests | ISO 13485 §8.2.6 – Product Monitoring and Measurement |
2. Sensor Calibration & Metrology Labs
Carejoy operates on-site Class 10,000 cleanroom sensor calibration laboratories, where each CMOS imaging sensor undergoes individual characterization using traceable NIST-equivalent standards. Calibration includes:
- Pixel Response Uniformity (PRNU): Correction mapping for sensor gain variation
- Dark Signal Non-Uniformity (DSNU): Thermal noise compensation at multiple exposure levels
- Modulation Transfer Function (MTF) Validation: Ensures ≥ 5.0 lp/mm resolution at 10% MTF
- Dose Linearity Testing: From 0.1 mGy to 5.0 mGy across 60–90 kVp spectra
AI-driven calibration software dynamically adjusts flat-field correction matrices in real time, ensuring clinical consistency across batches and environmental conditions.
3. Durability & Environmental Testing Protocols
To ensure clinical reliability, Carejoy subjects all DXUs to accelerated life testing simulating 7+ years of clinical use:
| Test Type | Parameters | Pass Criteria |
|---|---|---|
| Drop & Impact | 1.2m drop onto concrete (6 axes), 50 cycles | No sensor misalignment; image integrity maintained |
| Thermal Cycling | -10°C to +50°C, 200 cycles, 30 min dwell | No condensation; calibration retention ±2% |
| Vibration (Transport) | 5–500 Hz, 1.5g RMS, 3 axes, 4 hrs | No solder fractures or connector failure |
| Flex & Bite Simulation | 50,000 cycles at 50N lateral force (for intraoral sensors) | No cable fatigue or image artifacts |
| Autoclave Resistance (for handles) | 134°C, 2.1 bar, 18 min, 200 cycles | No warping or seal degradation |
4. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in digital dental hardware stems from a confluence of strategic advantages:
- Integrated Supply Chains: Proximity to semiconductor fabs, precision mold makers, and rare-earth magnet producers reduces BOM costs by 30–40% vs. EU/US counterparts.
- Advanced Automation: Shanghai and Shenzhen facilities deploy AI-guided SMT lines and robotic final assembly, minimizing human error and labor cost.
- Regulatory Agility: CFDA (NMPA) pathways are increasingly harmonized with global standards, enabling faster time-to-market without compromising quality.
- R&D Investment: Chinese medtech firms reinvest ~12% of revenue into R&D—focused on AI imaging, open interoperability (via DICOM and open APIs), and predictive maintenance.
- Economies of Scale: High-volume production of shared components (e.g., AI SoCs, wireless modules) across consumer and medical lines drives down unit costs.
Carejoy Digital leverages this ecosystem to deliver sub-€1,200 panoramic CBCT systems and €399 intraoral sensors with performance metrics rivaling premium European brands priced 2–3x higher.
Support & Digital Integration
Carejoy supports clinics and labs through:
- 24/7 Remote Technical Support with AR-assisted diagnostics
- Over-the-Air (OTA) Software Updates for AI scanning enhancements and DICOM 3.0 compliance
- Open Architecture Compatibility: Native export to STL/PLY/OBJ for seamless CAD/CAM and 3D printing workflows
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Digital Xray Unit.
✅ Open Architecture
Or WhatsApp: +86 15951276160
