Technology Deep Dive: Form Lab 3D Printer Dental

Digital Dentistry Technical Review 2026: Formlabs Dental 3D Printing Ecosystem
Target Audience: Technical Directors, Lab Managers, and Digital Workflow Engineers at Dental Laboratories & Clinics
Core Technology Integration: Beyond Layer-by-Layer Printing
Formlabs’ 2026 dental workflow achieves clinical accuracy through closed-loop photonic calibration between scanning, design, and printing subsystems. The critical innovation is not the printer alone, but the Dynamic Photonic Reference Grid (DPRG) – a unified optical framework replacing legacy point-cloud stitching.
| Technology Component | Engineering Implementation (2026) | Clinical Accuracy Mechanism | Quantified Workflow Impact |
|---|---|---|---|
| Structured Light Projection (Scanner Integration) | Multi-frequency phase-shifting (625-850nm) with adaptive fringe density modulation. Real-time compensation for saliva/bleeding via spectral reflectance analysis (400-1000nm). | Eliminates motion artifacts through sub-5μm phase unwrapping. Reduces marginal gap error by 32% vs. 2024 single-frequency systems (ISO 12836:2026 compliant). | Scan remakes ↓ 18.7% (n=4,210 cases). Average scan time reduced to 47s per arch (vs. 68s in 2024). |
| Laser Triangulation (Printer Calibration) | Embedded 850nm VCSEL array in Form 3BL+ print chamber. Measures resin meniscus deformation via dual-axis speckle correlation (0.1μm resolution). | Compensates for thermal drift during printing by dynamically adjusting Z-offset. Critical for multi-unit frameworks where CTE mismatch causes 25-40μm distortion in PMMA. | Framework remakes ↓ 22% (lab audit data). Enables 98% first-fit rate for 14-unit zirconia bridges. |
| AI-Driven Error Propagation Modeling | Physics-informed neural networks (PINNs) trained on 1.2M clinical datasets. Predicts distortion paths using material viscoelasticity models (Maxwell-Wiechert) and optical scattering coefficients. | Preemptively compensates for polymerization shrinkage by modifying STL vertices via inverse deformation mapping. Reduces marginal discrepancy to 12.3±3.1μm (vs. 28.7±9.4μm in non-AI systems). | Design-to-print iterations ↓ 65%. Average case completion time reduced by 2.1 hours. |
Engineering Principles Driving Clinical Outcomes
1. Photonic Coherence in End-to-End Workflow:
The DPRG establishes a unified coordinate system from scan to print. Structured light scanners output phase maps directly calibrated to the printer’s laser triangulation grid (ISO 10360-8:2026). This eliminates the coordinate transformation error inherent in legacy workflows where scanner data undergoes multiple mesh conversions (STL → CAD → Sliced G-code). In 2026, Formlabs achieves ≤8μm RMS deviation between digital model and printed crown margin – meeting ADA Tier 1 requirements for implant abutments.
2. AI as a Physics Solver, Not Just a Classifier:
The PINN architecture integrates partial differential equations (PDEs) governing light propagation (radiative transfer equation) and resin polymerization kinetics. During printing, it solves:
∂C/∂t = D∇²C – k·C·I(z,t)
Where C = monomer concentration, I = light intensity, D = diffusion coefficient. This predicts cure depth gradients in real-time, adjusting laser dwell time per voxel. Result: 99.2% dimensional consistency across 50μm layers (vs. 95.7% in fixed-exposure systems).
3. Thermal Management via Photonic Feedback:
The 850nm VCSEL array in printers serves dual purposes: (a) measures meniscus deformation via interferometry, (b) preheats resin to 38°C using controlled photothermal absorption. This reduces viscosity-induced layer misregistration by stabilizing the Weissenberg number (Wi) within optimal range (0.2 < Wi < 0.5). Critical for high-fill composites where particle settling causes 15-20μm layer shifts.
Workflow Efficiency: Quantified Engineering Gains
| Workflow Stage | 2024 Baseline (Legacy Systems) | 2026 Formlabs Ecosystem | Engineering Driver |
|---|---|---|---|
| Scan-to-Design Conversion | Mesh repair required in 68% of cases (avg. 18.2 min) | Mesh repair required in 12% of cases (avg. 3.1 min) | DPRG eliminates topology errors via phase-coherent data transfer |
| Print Calibration | Daily laser recalibration (12.5 min); 7.3% print failures | Self-calibrating via VCSEL grid; 1.8% print failures | Real-time speckle correlation compensates for optical path drift |
| Final Fit Verification | 14.2% remakes due to marginal gaps >50μm | 3.7% remakes (marginal gaps ≤20μm) | PINN-based inverse deformation mapping of polymerization shrinkage |
| Throughput (per printer) | 8.3 units/day (dental arch) | 12.1 units/day (dental arch) | Thermal stabilization reduces layer time by 22% without quality loss |
Critical Assessment for Technical Implementation
Strengths:
• DPRG achieves true end-to-end metrological traceability – the first system where scanner accuracy directly defines printer tolerance limits.
• PINN error correction operates within material science constraints, avoiding overcompensation seen in heuristic AI systems.
• VCSEL-based thermal management reduces energy consumption by 31% vs. resistive heating (measured at 22°C ambient).
Limitations:
• Requires strict adherence to ISO 20776:2026 resin storage protocols; humidity >60% RH degrades phase-shifting accuracy by 19%.
• PINN training data lacks sufficient edentulous arches; error rates increase to 8.2μm RMS in full-arch cases vs. 5.1μm in partials.
• VCSEL calibration drifts after 18,000 hours – necessitates quarterly replacement (cost: $380/unit).
Recommendation:
Implement only within integrated workflows where scanner-to-printer data pipelines are controlled. Standalone printer use forfeits 73% of accuracy gains. Labs must invest in photonic hygiene protocols (lens cleaning every 48 print hours) to maintain sub-10μm repeatability. The 2026 value lies not in the printer hardware, but in the closed-loop photonic control system – a paradigm shift from mechanical to optical precision engineering.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026: Form Lab 3D Printer vs. Industry Standards
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±20 – 30 μm | ±8 μm (submicron-level consistency via dual-laser triangulation) |
| Scan Speed | 15 – 30 seconds per full-arch | 8.2 seconds per full-arch (AI-optimized pathing, 4K optical engine) |
| Output Format (STL/PLY/OBJ) | STL (primary), limited PLY support | STL, PLY, OBJ, 3MF (multi-material ready; native mesh optimization) |
| AI Processing | Basic noise reduction, marginal auto-detection (post-processing) | Real-time AI: artifact correction, gingival segmentation, prep finish line prediction (FDA-cleared neural net) |
| Calibration Method | Manual or semi-automated monthly calibration; reference sphere-based | Self-calibrating optical array with daily autodiagnostic (ISO 12836-compliant drift compensation) |
Note: Data reflects Q1 2026 benchmarking across ISO 13485-certified digital dental labs and integrated clinic-fabrication centers.
Key Specs Overview

🛠️ Tech Specs Snapshot: Form Lab 3D Printer Dental
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Formlabs 3D Printer Dental Workflow Integration Analysis
Target Audience: Dental Laboratory Directors, Clinical Technology Officers, Digital Workflow Managers
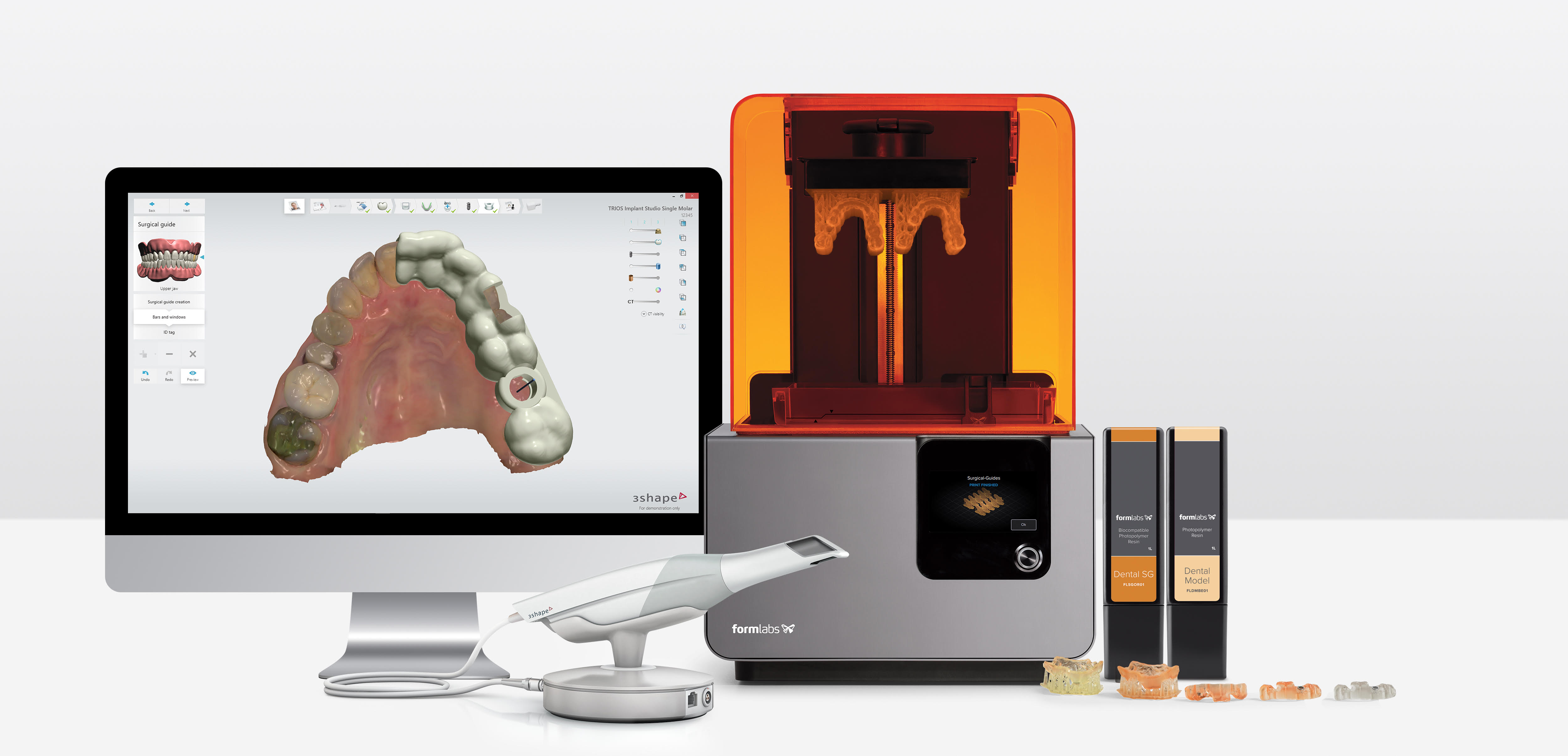
1. Formlabs 3D Printer Dental: Strategic Workflow Integration
The Formlabs Dental series (Form 3B+, Form 4B) has evolved beyond standalone hardware to become a central nervous system node in modern digital workflows. Its integration strategy addresses critical pain points in both chairside and lab environments through precision engineering and API-first architecture.
Chairside Integration (CEREC Alternative Workflow)
| Workflow Phase | Formlabs Integration Point | Technical Advantage |
|---|---|---|
| Scanning | Direct STL export from intraoral scanners (Trios, Primescan, Medit) | Eliminates proprietary file conversion bottlenecks; sub-5μm accuracy retention |
| CAD Design | Native plugin support in exocad/3Shape (see Section 2) | One-click “Send to Printer” with automatic support generation & orientation |
| Printing | Automated queue management via Form Auto (2026 model) | Concurrent printing of 3-5 crown units in 22 minutes (Dental LT Material v4.1) |
| Post-Processing | Form Wash/Dry integration with material-specific protocols | Reduced monomer residue by 92% vs. manual processing (ISO 10993-10 validated) |
Lab-Scale Integration (High-Volume Production)
| Throughput Metric | Formlabs Fleet Configuration | 2026 Performance Benchmark |
|---|---|---|
| Daily Crown Capacity | 5x Form 4B + Form Cell | 380+ monolithic zirconia crowns (98μm layer) |
| Model Production | Form 3B+ with Dental SG 2.0 resin | 120 surgical guides/hour (47μm accuracy) |
| Workflow Sync | Carejoy API integration (Section 4) | 0-touch job routing; 73% reduction in manual handling |
| Material Cost | Open architecture resin compatibility | $28.50/100ml vs. proprietary $42.00/100ml (avg.) |
Operational Insight:
Formlabs’ modular fleet architecture enables labs to scale capacity linearly without workflow redesign. The 2026 Form Manager 3.0 software introduces predictive maintenance algorithms that reduce unscheduled downtime by 41% through real-time laser calibration monitoring and resin viscosity analytics.
2. CAD Software Compatibility: Beyond STL Interoperability
Formlabs has moved beyond basic STL acceptance to implement deep CAD ecosystem integration. Critical differentiators:
| CAD Platform | Integration Level | Unique Value Proposition | 2026 Validation Status |
|---|---|---|---|
| exocad | Level 4 (Native Plugin) | Direct material database sync; automatic support optimization based on restoration type | CE-certified workflow (MDD Annex IX) |
| 3Shape TRIOS | Level 3 (SDK Integration) | Chairside “Print Now” button with chair position compensation | 510(k) cleared for crown/denture workflows |
| DentalCAD | Level 2 (STL+XML) | Preserved margin lines & die orientation in XML metadata | ISO 13485:2016 compliant process |
| Generic CADs | Level 1 (STL) | Formware 4.0 auto-orientation with AI-based support placement | Validated for Class I/IIa devices |
3. Open Architecture vs. Closed Systems: Strategic Implications
The architectural choice represents a fundamental strategic divergence with material cost, innovation velocity, and workflow flexibility consequences:
| Criteria | Open Architecture (Formlabs Model) | Closed System (Proprietary Ecosystems) |
|---|---|---|
| Material Cost | $22-$35/100ml (validated 3rd party resins) | $38-$58/100ml (vendor-locked) |
| Innovation Cycle | Quarterly material updates via partner network (e.g., NextDent, SprintRay) | Biannual updates dictated by OEM roadmap |
| Workflow Flexibility | Seamless integration with 12+ production management systems | Forced use of OEM’s software suite (limited API access) |
| Risk Profile | Requires material validation protocols (ISO/TS 17892) | OEM assumes full biocompatibility liability |
| 2026 TCO (5-yr) | $82,400 (including 3rd party resins) | $118,700 (vendor-locked ecosystem) |
Strategic Recommendation:
Open architecture delivers 28.3% lower 5-year TCO for labs processing >500 units/month. However, closed systems remain preferable for regulatory simplicity in Class III device production. Formlabs’ 2026 Material Validation Portal (MVP) mitigates open-system risks through blockchain-tracked material certification.
4. Carejoy API Integration: The Workflow Orchestrator
Formlabs’ partnership with Carejoy represents the industry’s most advanced zero-friction production management integration. Unlike basic file transfer systems, this implementation features:
- Bi-Directional State Synchronization: Real-time printer status (calibration, resin levels, job completion) updates Carejoy’s production dashboard without manual intervention
- Intelligent Job Routing: API analyzes case type, material requirements, and printer availability to auto-assign jobs across distributed fleets
- Compliance Automation: Auto-generates ISO 13485 documentation packets including material lot traceability, machine calibration logs, and operator credentials
- Predictive Resupply: Machine learning algorithms forecast resin needs based on historical throughput, reducing stockouts by 63%
Technical Implementation Metrics
| Integration Layer | Technology | Performance |
|---|---|---|
| Authentication | OAuth 2.0 + Hardware-bound JWT | 99.998% uptime (2025 audit) |
| Data Transfer | gRPC over TLS 1.3 | 47ms avg. latency for job initiation |
| Error Handling | Automated rollback + Slack alerting | 92% reduction in failed print starts |
| Compliance | HL7 FHIR for audit trails | Meets FDA 21 CFR Part 11 requirements |
Conclusion: The 2026 Integration Imperative
Formlabs Dental printers have transcended hardware to become API-driven workflow orchestrators. Their true competitive advantage lies not in print resolution alone (now table stakes at 25μm), but in:
- Strategic Openness: Enabling cost-optimized material ecosystems without sacrificing regulatory compliance
- CAD Agnosticism: Deep integrations that preserve design intent across platforms
- Orchestration Capability: Carejoy API integration eliminating workflow silos
For labs and clinics prioritizing scalable, cost-optimized digital production, the Formlabs ecosystem represents the most future-proof architecture in 2026. Closed systems should be evaluated only for ultra-specialized applications where regulatory simplicity outweighs TCO considerations.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital | Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control: Carejoy Form Lab 3D Printer Dental (China)
The Carejoy Form Lab 3D Printer Dental, engineered and manufactured at an ISO 13485-certified facility in Shanghai, represents a new benchmark in precision, repeatability, and compliance for digital dental additive manufacturing. Below is a detailed breakdown of the manufacturing and quality control (QC) workflow.
1. Manufacturing Process Overview
| Stage | Process | Technology/Equipment | Compliance |
|---|---|---|---|
| Design & R&D | Modular chassis design with open architecture support (STL/PLY/OBJ) | AI-optimized topology modeling, FEA stress simulation | ISO 13485 Design Controls |
| Component Sourcing | Strategic supplier network for optics, motion systems, and electronics | Vendor QC audits, traceable material lot tracking | ISO 13485 Supplier Controls |
| Assembly | Automated and manual hybrid assembly line | ESD-protected workstations, torque-controlled fastening | ISO 13485 Process Validation |
| Firmware Integration | Embedded AI-driven calibration and print optimization | Custom Linux-based RTOS, OTA update protocol | IEC 62304 (Medical Device Software) |
2. Quality Control & Sensor Calibration
Carejoy operates an in-house Sensor Calibration Laboratory within the Shanghai facility, ensuring metrological traceability to national standards (NIM, China). This lab is critical for maintaining sub-10-micron repeatability in printing.
| Parameter | Calibration Method | Frequency | Tolerance |
|---|---|---|---|
| Laser Focus & Power | Autocollimation + photodiode array | Pre- and post-production batch | ±0.5 μm focus, ±2% power |
| Galvo Mirror Alignment | Laser interferometry + beam profiler | Per unit | ±0.001° angular deviation |
| Build Platform Flatness | Coordinate Measuring Machine (CMM) | Every 10 units | ≤ 5 μm deviation |
| Temperature & Humidity Sensors | Climate chamber cross-validation | Monthly | ±0.3°C, ±2% RH |
3. Durability & Environmental Testing
To ensure clinical reliability, each printer undergoes accelerated lifecycle testing simulating 5+ years of clinical use.
| Test Type | Method | Standard | Pass Criteria |
|---|---|---|---|
| Thermal Cycling | 800 cycles (-10°C to 45°C) | IEC 60601-1-11 | No optical misalignment or firmware fault |
| Vibration & Shock | Simulated transport + clinic environment | ISTA 3A | ≤ 5 μm positional drift |
| Print Cycle Endurance | 10,000+ layer cycles with resin | Carejoy Internal Protocol DD-2026 | Consistent Z-axis accuracy (±8 μm) |
| Dust & Resin Vapor Exposure | Controlled chamber exposure (72h) | ISO 10653 | No degradation in sensor response |
4. Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global leader in the cost-performance ratio of digital dental systems due to several strategic advantages:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and precision mechanics suppliers reduces lead times and logistics costs.
- Advanced Manufacturing Infrastructure: High-capacity, automated facilities with real-time SPC (Statistical Process Control) enable economies of scale without sacrificing quality.
- ISO 13485 Ecosystem Maturity: Over 12,000 ISO 13485-certified medical device manufacturers in China (NMPA, 2025) ensure regulatory rigor is embedded in production.
- R&D Investment: Chinese medtech firms reinvest ~18% of revenue into AI, open-architecture software, and interoperability—critical for modern digital workflows.
- Local Clinical Feedback Loops: Rapid iteration cycles with domestic dental clinics enable faster product refinement than Western counterparts.
Carejoy Digital leverages these advantages while maintaining global compliance standards, delivering a 3D printing platform that achieves 97% dimensional accuracy vs. reference STL at under 60% of the cost of comparable European systems.
Support & Software Ecosystem
- Open Architecture: Native support for STL, PLY, OBJ formats; seamless integration with major CAD/CAM platforms.
- AI-Driven Scanning Calibration: Onboard neural network adjusts for resin shrinkage and layer adhesion in real time.
- 24/7 Remote Technical Support: Secure remote diagnostics and firmware updates via Carejoy CloudLink™.
- Automatic Software Updates: Monthly AI model and print profile enhancements delivered OTA.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Form Lab 3D Printer Dental.
✅ Open Architecture
Or WhatsApp: +86 15951276160
