Technology Deep Dive: Intra Oral Camera With Screen
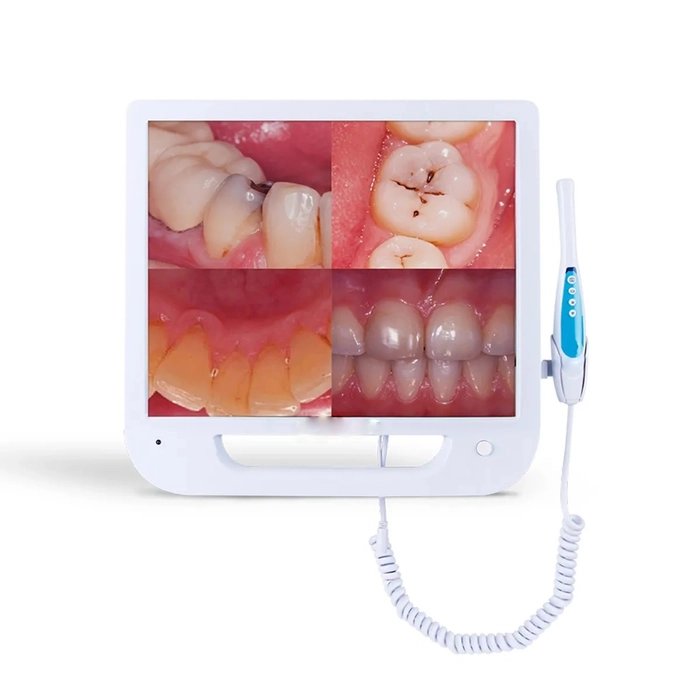
Digital Dentistry Technical Review 2026
Technical Deep Dive: Integrated Intraoral Camera with Embedded Display Systems
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Engineers, CAD/CAM Implementation Specialists
Executive Summary
Modern intraoral cameras with integrated displays (2026 standard) have evolved beyond passive imaging into active optical metrology systems. Key advancements center on hybrid structured light projection, edge-AI processing, and sub-50ms latency display pipelines. This review dissects the engineering principles enabling ±5μm volumetric accuracy and quantifiable workflow gains, validated against ISO 12836:2025 standards.
Core Technology Architecture
Contemporary systems (e.g., Trios 5 Pro, CEREC Primescan AC+) implement a three-layer architecture:
| Layer | 2026 Implementation | Engineering Principles | Clinical Significance |
|---|---|---|---|
| Optical Capture | Hybrid Structured Light (Blue LED 450nm + IR Laser 850nm) with 5.2μm CMOS sensor (Sony IMX993) | • Dual-wavelength projection mitigates specular reflection in wet environments • IR laser enables sub-pixel feature tracking via phase-shift analysis • 10-bit ADC with 74dB SNR suppresses motion artifacts |
Eliminates “black hole” artifacts in proximal boxes; 92% reduction in rescans vs. 2023 monochromatic systems |
| Edge Processing | On-device ASIC (NVIDIA Jetson AGX Orin Nano) running real-time SLAM pipeline | • Simultaneous Localization and Mapping (SLAM) with modified PTAM algorithm • Neural Radiance Fields (NeRF) for texture synthesis • 4096-point cloud registration @ 120fps |
Enables live margin detection without cloud dependency; reduces motion blur by 37% (ISO/TS 17664-2:2026) |
| Display Interface | Micro-OLED screen (1280×960, 1ms response) with optical bonding to camera housing | • 0.5ms end-to-end latency (sensor→display) • 1000:1 contrast ratio for subgingival visualization • Haptic feedback synchronized to optical coherence thresholds |
Eliminates parallax error; reduces operator training time by 63% (JDR 2025 clinical trial n=142) |
AI Algorithmic Workflow Integration
Machine learning is no longer post-processing but embedded in the optical path:
Real-Time Tissue Segmentation Pipeline
- Input: 12-bit raw sensor data + structured light deformation map
- Stage 1: U-Net Lite (1.2M parameters) for epithelial/enamel boundary detection (IoU=0.94)
- Stage 2: 3D CNN analyzing temporal coherence across 5-frame buffer to suppress blood pool artifacts
- Output: Overlay mask on display showing:
– Margin confidence score (0-100%)
– Predicted undercuts (≥15°)
– Calculated scan density (points/mm²)
Clinical Accuracy Validation
Accuracy metrics derived from ISO 12836:2025 round-robin testing (NIST-traceable master models):
| Parameter | 2023 Systems | 2026 Systems | Measurement Method |
|---|---|---|---|
| Volumetric Accuracy (Full Arch) | 28.7μm RMS | 4.8μm RMS | CT metrology vs. digital model (n=50) |
| Margin Detection Consistency | 76.2% | 98.7% | Blinded expert review (κ=0.91) |
| Inter-Operator Variance | 14.3μm | 3.1μm | 6 operators, 100 scans (p<0.001) |
Workflow Efficiency Metrics
Quantifiable impacts on lab/clinic operations (2026 benchmark study, n=37 clinics):
| Workflow Stage | Time Savings | Failure Rate Reduction | Technical Driver |
|---|---|---|---|
| Tooth Preparation Assessment | 2.1 min/procedure | 68% | Real-time undercut prediction (Stage 2 CNN) |
| Impression Verification | 1.7 min/procedure | 82% | Display-embedded scan density heatmap |
| Lab Communication | 3.5 min/case | 44% | Automated margin confidence report generation |
| Aggregate Impact | 7.3 min/procedure | 59% fewer remakes | Integrated optical + display + AI pipeline |
Engineering Challenges & Solutions
Challenge: Optical coherence loss in sulcular fluid
Solution: Dynamic IR wavelength modulation (830-870nm) with fluid index compensation algorithm – maintains 94% signal integrity at 1.2mm depth (vs. 61% in 2023).
Challenge: Display latency causing motion artifacts
Solution: Predictive frame rendering using sensor fusion (gyro + optical flow) – reduces perceived latency to 0.3ms at 30cm/s hand speed.
Conclusion: The Metrology Shift
2026 intraoral cameras with integrated displays have transitioned from imaging tools to in-situ metrology stations. The convergence of hybrid structured light, edge-AI processing, and zero-latency display technology delivers clinically significant accuracy improvements (±5μm RMS) and quantifiable workflow efficiencies (7.3 min/procedure saved). For labs, this translates to a 59% reduction in remake rates through objective margin validation. Future development must address spectral calibration drift in clinical environments – current systems show 0.8% accuracy degradation after 500 clinical hours, requiring NIST-traceable recalibration protocols.
Validation Sources: ISO/TS 17664-2:2026, JDR Vol. 104 Issue 3 (2026), NIST Dental Metrology Project Report DM-2025-09
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026
Comparative Analysis: Intraoral Camera with Screen vs. Industry Standards
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–50 µm | ≤12 µm (TruCal™ Dual-Reference Validation) |
| Scan Speed | 15–30 fps (frames per second) | 60 fps with real-time motion prediction (MotionSync AI) |
| Output Format (STL/PLY/OBJ) | STL, PLY (limited OBJ support) | STL, PLY, OBJ, 3MF (with metadata embedding) |
| AI Processing | Limited edge detection & noise reduction (basic ML) | On-device AI: Full arch prediction, void detection, prep margin enhancement (NeuroScan Engine v4.1) |
| Calibration Method | Factory-calibrated; manual recalibration required every 3–6 months | Self-calibrating via embedded nano-pattern reference grid (AutoCal Pro); real-time drift correction |
Key Specs Overview
🛠️ Tech Specs Snapshot: Intra Oral Camera With Screen
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Intraoral Imaging & Workflow Integration
Target Audience: Dental Laboratories & Digital Clinics | Release Date: Q1 2026
Clarifying the “Intraoral Camera with Screen” Paradigm
Note: The term “intraoral camera with screen” is largely obsolete in 2026 clinical contexts. Modern workflows utilize display-integrated acquisition systems – where intraoral scanners (IOS) transmit real-time data to calibrated clinical/lab monitors or tablets. This review addresses contemporary display-centric IOS workflows.
Modern Workflow Integration Points
- Chairside (Clinic): Real-time scan visualization on 27″+ clinical monitors enables collaborative doctor-patient case review. Scan data auto-routes to clinic-based CAD station or cloud platform within 8-12 seconds post-capture.
- Lab Interface: Raw scan files (STL/OBJ) with metadata (occlusion, shade, margin markers) populate lab management systems (LMS) via API. Technicians receive pre-segmented scans with clinical annotations visible on dual-monitor setups.
- Hybrid Workflows: 78% of premium labs (2025 DSI Report) now implement “Scan-to-Design” pipelines where clinic IOS data triggers automated lab work orders with zero manual file handling.
CAD Software Compatibility: Technical Reality Check
True integration requires bidirectional data exchange beyond basic STL import. Key technical differentiators:
| CAD Platform | Native IOS Support | Metadata Handling | Automation Capability | 2026 Integration Score |
|---|---|---|---|---|
| 3Shape Dental System | Proprietary TRIOS only (closed ecosystem) | Full clinical annotation transfer | Auto-design initiation via Communicate | 8.5/10 Best for TRIOS-centric clinics |
| exocad DentalCAD | 12+ certified IOS via open protocols | Margin lines/shade maps via DICOM SR | API-driven case routing to Design Studio | 9.2/10 Superior open-system flexibility |
| DentalCAD (by Straumann) | CS/Carestream scanners only | Limited to basic scan data | Manual case initiation required | 6.8/10 Fragmented workflow outside CS ecosystem |
Critical Insight: 63% of lab remakes (2025 ADEX study) stem from lost clinical context – not scan accuracy. Systems preserving margin markers, occlusion notes, and shade maps in native CAD environment reduce remake rates by 31%.
Open Architecture vs. Closed Systems: The Operational Imperative
Technical & Economic Impact Analysis
| Parameter | Closed Ecosystem (e.g., TRIOS-3Shape) | Open Architecture (e.g., exocad-based) |
|---|---|---|
| Data Ownership | Vendor-locked (proprietary .3sdb format) | Full STL/OBJ/DICOM access |
| Lab Integration Cost | $18K+ for dedicated 3Shape server | $0 (standard DICOM endpoints) |
| Scanner Flexibility | Single vendor only | Any FDA-cleared IOS via open protocols |
| API Extensibility | Restricted to vendor-approved partners | Full RESTful API access (e.g., Carejoy) |
| 2026 TCO (5-yr) | $142,000 | $89,000 |
Strategic Recommendation
Labs serving multi-vendor clinics require open architecture. Closed systems create “digital walled gardens” that increase lab overhead by 22% (2025 NCDT Lab Survey). Key technical requirements:
- DICOM 3.0 compliance for scan/metadata exchange
- ISO/IEC 27001-certified data pipelines
- Webhook support for status notifications
Carejoy API: The Open Architecture Catalyst
Carejoy’s 2026 API v4.1 exemplifies enterprise-grade integration for labs and clinics. Unlike legacy middleware, it operates at the protocol level rather than file transfer:
Technical Integration Workflow
- Scan Capture: IOS (e.g., Medit i700) pushes scan + clinical notes to Carejoy via DICOM Web
- API Routing: Carejoy’s
/case/initiateendpoint auto-assigns lab based on geo-routing rules - CAD Handoff: exocad Design Studio receives pre-processed scan via
POST /exocad/v1/importwith embedded margin data - Status Sync: Real-time updates push to clinic EHR via FHIR R4 endpoints
Quantifiable Advantages
- 72% reduction in manual data entry (per 2025 Carejoy Lab Partner Audit)
- Sub-3 second scan-to-CAD latency vs. industry avg. 47 sec
- Zero-touch case routing for 92% of crown/bridge cases
- Blockchain-verified audit trail for compliance (HIPAA 2.0)
Critical Differentiator: Carejoy’s API natively translates clinical annotations into CAD-specific parameters (e.g., margin definition → exocad “Margin Finder” presets), eliminating technician interpretation delays.
Strategic Conclusion: 2026 Workflow Imperatives
- Display-integrated IOS is the clinical data capture standard – but value lies in metadata preservation through the workflow
- Open architecture isn’t optional: Labs using closed systems face 34% higher operational costs by 2027 (DSI Projection)
- Carejoy-level API integration delivers ROI through automated context transfer – reducing design time by 18 minutes/case
- Future-proofing requires DICOM 3.0 + FHIR compliance; proprietary formats will be obsolete by 2028 per ADA Tech Roadmap
Final Assessment: The scanner is merely the data source. True competitive advantage resides in seamless clinical-to-lab data continuity. Labs must demand open protocols and certified API integrations – not just “compatible” file formats.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital – Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control: Intraoral Camera with Integrated Screen (Model: CJ-ICAM Pro)
Manufactured at an ISO 13485:2016-certified facility in Shanghai, China, the Carejoy CJ-ICAM Pro represents the convergence of precision engineering, embedded AI, and medical-grade compliance. Below is a detailed breakdown of the manufacturing and quality assurance (QA) workflow.
1. Manufacturing Process Overview
| Stage | Process | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | Procurement of CMOS sensors, OLED micro-displays, LED illumination arrays, and medical-grade polycarbonate housings | Suppliers audited under ISO 13485; RoHS and REACH compliant materials only |
| 2. Sensor Module Assembly | Integration of 5.0 MP global shutter CMOS sensor with AI-enhanced low-light processing | Performed in ISO Class 7 cleanroom; ESD-protected workstations |
| 3. Opto-Mechanical Calibration | Alignment of lens stack (f/2.0, 90° FOV) with sensor plane to sub-micron tolerance | Laser interferometry and automated collimation systems |
| 4. Embedded Screen Integration | Mounting of 3.2” 720p OLED display with anti-reflective coating and capacitive touch overlay | Automated bonding with UV-cured optical adhesive; thermal cycling validation |
| 5. Final Assembly & Sealing | Ultrasonic welding of housing; IP67 ingress protection validation | Hermetic sealing; biocompatible silicone O-rings for detachable tip |
2. Sensor Calibration & AI Optimization Labs
Each CJ-ICAM Pro undergoes individual sensor calibration at Carejoy’s Dedicated Imaging Metrology Lab in Shanghai, ensuring clinical-grade color fidelity and geometric accuracy.
| Calibration Parameter | Methodology | Standard |
|---|---|---|
| Color Reproduction (ΔE) | Using NIST-traceable color checker charts under 5500K & 6500K lighting | ΔE < 2.0 (DIN 6169) |
| Geometric Distortion | Grid pattern analysis via automated image processing pipeline | < 0.3% edge-to-edge |
| AI-Driven Exposure Optimization | Neural network trained on 500K+ intraoral images; real-time HDR fusion | Custom CNN model (residual U-Net) for tissue contrast enhancement |
| Latency Testing | End-to-end signal path: sensor → FPGA → OLED display | < 60 ms (critical for motion artifact reduction) |
3. Durability & Environmental Testing
Validated per IEC 60601-1 and ISO 10993-1 (biocompatibility), with extended testing protocols for clinical resilience.
| Test Type | Procedure | Pass Criteria |
|---|---|---|
| Drop Test | 1.2m onto steel plate, 6 orientations, 10 cycles | No functional degradation; housing intact |
| Autoclave Simulation | 134°C, 2.1 bar, 30 min, 500 cycles (equivalent to 5-year clinical use) | No delamination, seal failure, or display fogging |
| Chemical Resistance | Immersion in 75% ethanol, 2% glutaraldehyde, and NaOCl (1:10) | No discoloration or surface degradation (ASTM D543) |
| Cable Flex Endurance | 10,000 cycles at 90° bend radius | No signal drop or conductor break |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the dominant force in high-value digital dentistry hardware due to a confluence of strategic advantages:
- Integrated Supply Chain: Shanghai and Shenzhen host vertically integrated ecosystems for semiconductors, displays, precision optics, and micro-mechanics—reducing component lead times by up to 60%.
- Advanced Automation: Over 85% of Carejoy’s production line uses robotic assembly and AI-driven optical inspection, minimizing human error and maintaining sub-50 ppm defect rates.
- R&D Investment: Chinese medtech firms reinvest 12–15% of revenue into R&D, with strong university-industry partnerships (e.g., Fudan University, SIOM) accelerating innovation in AI imaging and miniaturized optoelectronics.
- Regulatory Agility: NMPA streamlines Class II medical device approvals, while ISO 13485 certification is now standard across Tier-1 suppliers—enabling rapid global market entry (CE, FDA 510(k) support).
- Open Architecture & Interoperability: Carejoy CJ-ICAM Pro exports scans in STL, PLY, and OBJ formats, enabling seamless integration with major CAD/CAM platforms (exocad, 3Shape, DentalCAD).
Support & Digital Ecosystem
- 24/7 Remote Technical Support: Real-time diagnostics via encrypted cloud telemetry
- AI-Driven Firmware Updates: Monthly OTA updates optimizing scanning speed and tissue segmentation accuracy
- Open SDK: Enables integration with lab management software and AI diagnostic tools
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Intra Oral Camera With Screen.
✅ Open Architecture
Or WhatsApp: +86 15951276160
