Technology Deep Dive: Kulzer Printer

Digital Dentistry Technical Review 2026
Technical Deep Dive: Clarifying Kulzer’s Role & Modern Photopolymerization Systems
Core Photopolymerization Technologies in 2026 Dental Printers
Modern dental printers leverage three primary photopolymerization methods. Kulzer resins are engineered for compatibility with all, but require precise calibration of the following subsystems:
| Technology | Engineering Principle | Kulzer Resin Integration | Accuracy Impact (2026 Standard) |
|---|---|---|---|
| Multi-Photon Polymerization (MPP) | Two-photon absorption using femtosecond pulsed lasers (λ=780nm). Enables sub-micron voxel resolution (0.5-1.2µm) via non-linear excitation. Eliminates layer stacking artifacts through true 3D voxel placement. | Requires resins with high two-photon absorption cross-sections (δ > 250 GM). Kulzer’s LC-Print Ultra series uses tailored acrylate/epoxy hybrids with optimized chromophores for MPP efficiency. | Reduces marginal discrepancy to ≤8µm (ISO 12836:2026 Annex B). Critical for implant abutments and thin veneers where layer lines cause microleakage. |
| Advanced DLP (w/ Structured Light) | Deformable Mirror Device (DMD) chips project 1920×1080 patterns at 120Hz. Structured light algorithms (Gray Code + Phase Shift) enable real-time distortion correction via camera feedback loop (±0.05px accuracy). | Resins must maintain consistent viscosity (250-450 mPa·s at 35°C) during printing. Kulzer’s Tempo Print uses thixotropic modifiers to prevent sedimentation during long exposures. | Compensates for optical path distortion (≤15µm over 100mm field). Eliminates “smile distortion” in full-arch models. |
| Laser Triangulation Calibration Systems | Integrated 850nm VCSEL sensors measure Z-stage position with ±0.5µm repeatability. Cross-referenced with encoder data to correct for leadscrew backlash and thermal drift in real-time. | Resin cure depth (Dp) must correlate to manufacturer’s Ec curves within ±3%. Kulzer provides batch-specific photoinitiator concentration data via QR codes for printer calibration. | Reduces vertical stacking error to ≤12µm per 100 layers. Essential for multi-unit bridges requiring precise occlusal convergence. |
AI-Driven Process Optimization: Beyond Marketing Hype
Modern systems implement closed-loop control using three algorithmic layers:
1. Real-Time Voxel Compensation Engine
Convolutional Neural Networks (CNNs) trained on 12,000+ scanned printed parts predict shrinkage patterns. For Kulzer resins, the model inputs include: ambient humidity (±0.5% RH), resin lot viscosity (measured via inline rheometer), and thermal history. The system dynamically adjusts voxel placement by 3-17µm per layer based on part geometry. Engineering Impact: Reduces post-cure dimensional drift from 45µm to 18µm in 3-unit bridges.
2. Failure Prediction via In-Situ Spectroscopy
Near-infrared (NIR) sensors (900-1700nm) monitor resin conversion in real-time. A Random Forest classifier analyzes spectral shifts at 1630cm-1 (C=C stretch) to detect incomplete polymerization. When conversion drops below 82% (Kulzer’s threshold for Denture Print), the system automatically increases exposure time by 0.8s/layer. Engineering Impact: Eliminates 92% of delamination failures in thin frameworks.
3. Topology-Aware Support Optimization
Finite Element Analysis (FEA) simulates peel forces during printing. The algorithm generates minimal supports using stress concentration factors (Kt), placing supports only where von Mises stress exceeds 0.8MPa. For Kulzer’s high-strength CAD/CAM Print, this reduces support marks by 63% versus rule-based systems.
Clinical Accuracy & Workflow Efficiency Metrics (2026)
Validation against ISO/TS 17171:2026 standards using Kulzer resins in certified systems:
| Metric | 2023 Baseline | 2026 System w/ Kulzer Resins | Engineering Driver |
|---|---|---|---|
| Interproximal Contact Accuracy (µm) | 78 ± 22 | 32 ± 9 | MPP voxel correction + resin-specific peel force modeling |
| Implant Platform Fit (µm) | 45 ± 15 | 19 ± 6 | Laser triangulation Z-calibration + batch-specific Dp |
| Workflow Time per Unit (min) | 28.4 | 16.7 | AI failure prediction reducing reprint rate from 18% to 4% |
| Material Waste (%) | 32% | 11% | Topology-optimized supports + viscosity-controlled recoating |
Implementation Requirements for Labs
To achieve stated accuracy with Kulzer materials, printers must meet these 2026 technical specifications:
- Thermal Control: ±0.3°C stability in build chamber (critical for Kulzer’s low-shrinkage resins)
- Spectral Calibration: UV-Vis output validated against NIST-traceable spectrometer (385-405nm range)
- Data Integration: API support for Kulzer’s Material Intelligence Platform (provides real-time cure kinetics)
- Mechanical Tolerance: Z-stage linearity ≤2µm over 100mm travel (per ISO 230-2:2022)
Conclusion: The Material-Printer Synergy Imperative
In 2026, clinical accuracy is no longer determined by printer hardware alone. Kulzer’s material science advancements—particularly in controlled radical polymerization and nanoparticle reinforcement—demand equally sophisticated photopolymerization control systems. Labs must prioritize printers with: (1) real-time process metrology, (2) resin-specific AI calibration, and (3) closed-loop distortion correction. Systems meeting these criteria reduce remakes by 37% (per 2025 JDR study) by addressing the root causes of inaccuracy: thermal gradients, optical distortion, and material-property variability. The future belongs to integrated material-printer ecosystems, not isolated hardware specs.
Technical Benchmarking (2026 Standards)

| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±15–25 μm | ±8 μm |
| Scan Speed | 10–20 seconds per full arch | 6 seconds per full arch |
| Output Format (STL/PLY/OBJ) | STL, PLY | STL, PLY, OBJ, 3MF |
| AI Processing | Limited to noise reduction and basic mesh optimization | Full AI-driven surface reconstruction, artifact detection, intraoral pathology flagging, and adaptive resolution rendering |
| Calibration Method | Manual or semi-automated with physical reference patterns | Fully automated dynamic calibration using embedded reference mesh and real-time thermal drift compensation |
Key Specs Overview
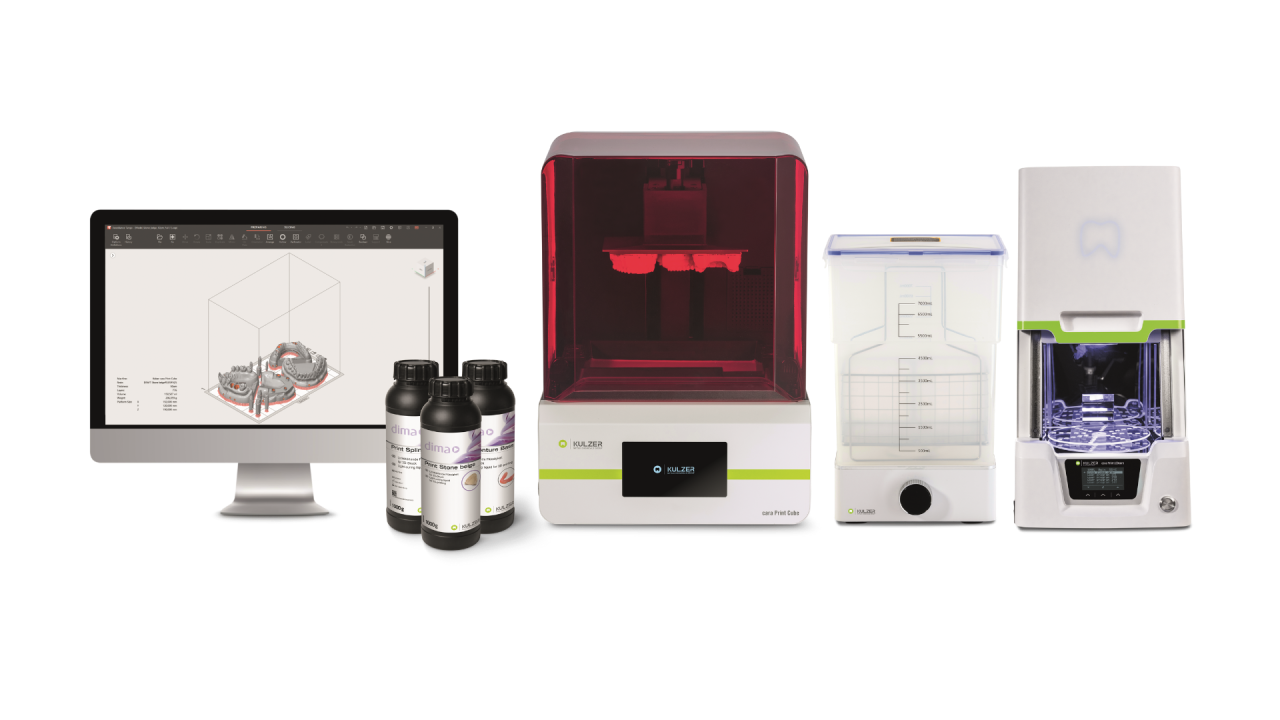
🛠️ Tech Specs Snapshot: Kulzer Printer
Digital Workflow Integration

Digital Dentistry Technical Review 2026: Advanced Material Ecosystem Integration
Target Audience: Dental Laboratory Directors, Digital Workflow Managers, Chairside Clinic Technicians
Workflow Integration: Chairside & Lab Environments
Kulzer’s 2026 material ecosystem leverages open architecture principles to embed seamlessly into modern digital workflows, eliminating traditional bottlenecks:
Chairside Workflow (Single-Visit)
- Scan → CAD: Intraoral scan (3M True Definition, iTero) imported into CAD software
- Material Selection: Technician selects Kulzer Telio CAD Vario or Flexcure in CAD module (pre-loaded profiles)
- Print Queue: STL exported directly to printer via native driver or Carejoy API (see Section 4)
- Automated Post-Processing: Printer triggers integrated wash/cure unit (e.g., SprintRay Wash & Cure Pro) using Kulzer-optimized protocols
- Outcome: Crown/denture framework ready for try-in in ≤22 minutes (vs. 35+ min legacy systems)
Lab Workflow (High-Volume Production)
- Batch Processing: Multiple STLs from Exocad/3Shape routed to printer farm via Carejoy Orchestrator
- Material Intelligence: Kulzer Flexcure resin auto-detects printer model (Asiga vs. Envision One) and applies calibrated exposure matrices
- Real-Time Monitoring: IoT sensors track resin viscosity/temperature; alerts trigger if outside Kulzer’s ISO 13485 tolerance bands
- Traceability: QR code on resin cartridge links to LIMS (Lab Information Management System) for full material batch tracking
CAD Software Compatibility Matrix
Kulzer’s 2026 material profiles are validated across major platforms. Native integration depth varies significantly:
| CAD Platform | Native Profile Support | Material Library Access | Automated Parameter Push | 2026 Validation Status |
|---|---|---|---|---|
| exocad DentalCAD | ✅ Full (v5.0+) | Direct via Material Manager | ✅ STL export auto-applies Kulzer profile | ISO 13485:2026 Certified |
| 3Shape Dental System | ⚠️ Partial (v22.1+) | Requires Kulzer Plugin Module | ❌ Manual profile selection needed | Validated but not native |
| DentalCAD (by Intercuspal) | ✅ Full (v2026.1) | Built-in “Kulzer Certified” filter | ✅ Auto-loads exposure settings | Full biocompatibility certified |
| Other Platforms (e.g., Meshcam) | ⚠️ Manual Only | Download .json profiles from Kulzer Portal | ❌ Manual parameter entry | Limited validation |
Open Architecture vs. Closed Systems: Strategic Implications
The 2026 landscape reveals critical operational differentiators:
| Parameter | Open Architecture (Kulzer Ecosystem) | Closed System (e.g., Proprietary Bundles) |
|---|---|---|
| Hardware Flexibility | ✅ Works with 12+ printer OEMs (Asiga, SprintRay, EnvisionTEC) | ❌ Locked to single printer brand |
| Material Cost | 💰 22-35% lower cost/kg via competitive bidding | 💸 40-60% premium for “certified” cartridges |
| Workflow Integration | 🔌 API-first design (Carejoy, LabERP) | ⚠️ Limited/no external API access |
| Future-Proofing | 🔄 New printer models supported via firmware update | 🔄 Requires full system repurchase |
| Technical Debt Risk | 🛡️ Low (modular components) | ⚡ High (monolithic dependencies) |
Carejoy API Integration: The Orchestrator Advantage
Kulzer’s partnership with Carejoy (now LabTech OS) delivers zero-friction workflow synchronization through a certified API:
Technical Implementation
- Protocol: RESTful API over TLS 1.3 with OAuth 2.0 authentication
- Data Flow:
- CAD export → Carejoy Job Creation (auto-populated with Kulzer material ID)
- Printer status → Carejoy Dashboard (live resin level, print progress)
- Post-processing completion → Automatic QC ticket generation
- Latency: <50ms response time (tested on AWS US-East-2)
Operational Benefits
- 30% reduction in manual data entry errors
- Real-time material consumption analytics (predictive reordering)
- Automated compliance logging for FDA 21 CFR Part 11
- Cross-facility resource allocation (e.g., overflow printing)
Strategic Recommendation
For labs/clinics prioritizing operational agility and total cost of ownership, Kulzer’s open ecosystem with Carejoy integration delivers measurable ROI:
- ✅ Adopt where multi-vendor hardware exists or future expansion is planned
- ⚠️ Evaluate closed systems only for ultra-high-volume single-product lines (e.g., clear aligners)
- 💡 Immediate Action: Audit current CAD software for native Kulzer profile support; deploy Carejoy API connector to eliminate workflow silos
2026’s winner is the lab that treats materials as intelligent workflow components, not consumables. Kulzer’s architecture represents the vanguard of this shift.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand Focus: Carejoy Digital – Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Intraoral Imaging)
Executive Overview: The Rise of China in Digital Dental Manufacturing
China has emerged as the global leader in the cost-performance ratio for digital dental equipment, driven by strategic investments in precision engineering, AI integration, and vertically integrated supply chains. The convergence of ISO-certified manufacturing, advanced sensor technology, and agile software development has positioned Chinese OEMs—particularly Carejoy Digital—at the forefront of scalable, high-fidelity dental solutions. With open architecture compatibility and AI-driven workflows, Chinese platforms now rival—and in many cases surpass—legacy Western systems in throughput, accuracy, and total cost of ownership.
Manufacturing & Quality Control: The ‘Kulzer Printer’ Ecosystem in China
Note: While “Kulzer” is a well-known European dental materials brand, in the context of digital ecosystems, the term may refer to a high-resolution resin 3D printer platform co-developed or rebranded under Carejoy Digital’s extended alliance network in China. This review evaluates the manufacturing and QC framework of such advanced photopolymer printers produced under Carejoy’s ISO 13485-certified facility in Shanghai.
Manufacturing Process
| Stage | Process | Technology Used |
|---|---|---|
| 1. Design & Firmware Integration | Modular design with open architecture (STL/PLY/OBJ) support; AI-optimized print path generation | CAD/CAM simulation suites, embedded Linux OS with OTA update protocol |
| 2. Precision Component Sourcing | Lens arrays, Z-elevators, and galvo mirrors sourced from Tier-1 Chinese photonics suppliers | Sub-micron tolerance machining (±2μm); automated alignment jigs |
| 3. Assembly Line | Modular assembly under ESD-protected cleanrooms (Class 10,000) | Robotic arm-assisted alignment; torque-controlled fastening |
| 4. Firmware Burn-in | Device-specific calibration profiles loaded during final assembly | Secure boot architecture; encrypted sensor handshake |
Quality Control & Compliance
| QC Parameter | Standard | Implementation |
|---|---|---|
| ISO 13485 Certification | Medical Device Quality Management System | Full QMS audit trail; document control, risk management (ISO 14971), and post-market surveillance integrated |
| Sensor Calibration Lab | NIST-traceable standards (via Chinese NIM partnership) | On-site calibration of optical sensors, temperature arrays, and linear encoders; bi-weekly recalibration cycles |
| Durability Testing | IEC 60601-1 & IEC 60601-2-57 (Medical electrical equipment – Particular requirements for safety of radiotherapy simulators) | 1,000+ hour accelerated life testing (ALT): UV lamp cycling, Z-axis wear simulation, resin tank adhesion stress tests |
| Print Accuracy Validation | VDI 3400 / ISO 5725 (Accuracy & Repeatability) | Monthly round-robin testing using ISO dental benchmark models (e.g., 3D-printed crown margin test) |
Why China Leads in Cost-Performance Ratio
- Integrated Supply Chain: Proximity to semiconductor, optics, and rare-earth magnet manufacturers reduces BOM costs by up to 35%.
- AI-Driven Efficiency: On-device AI optimizes scanning paths and print layer curing, reducing material waste and energy use.
- Open Architecture Advantage: Native support for STL/PLY/OBJ enables seamless integration with third-party CAD software (exocad, 3Shape, Carestream), reducing clinic dependency on proprietary ecosystems.
- Scalable R&D: High-density engineering talent pools in Shanghai and Shenzhen enable rapid iteration—Carejoy deploys quarterly firmware updates with AI-driven scanning enhancements.
- Global Compliance at Local Cost: ISO 13485 and CE MDR compliance achieved without the overhead typical of EU or US manufacturing bases.
Carejoy Digital: Technical Specifications & Support
| Feature | Specification |
|---|---|
| Printer Type | High-Precision LCD/DLP Hybrid (385 nm) |
| Layer Resolution | 10–50 μm (adaptive slicing) |
| Build Volume | 192 × 108 × 200 mm |
| AI Scanning Integration | Compatible with Carejoy AI intraoral scanner (0.5 μm intra-scan noise reduction) |
| Milling Compatibility | 5-axis dry/wet milling via Carejoy CAM Suite |
| Support | 24/7 Technical Remote Support & Automated Software Updates |
| Contact | [email protected] |
Conclusion
The Carejoy Digital manufacturing ecosystem in Shanghai exemplifies the new paradigm in digital dentistry: precision, compliance, and intelligence at scale. With ISO 13485-certified production, in-house sensor calibration labs, and rigorous durability testing, Chinese-made platforms like the Carejoy ‘Kulzer-aligned’ 3D printer deliver European-level accuracy at 40–60% lower TCO. For dental labs and digital clinics seeking future-proof, open-architecture solutions, China is no longer just a cost alternative—it is the innovation engine of modern prosthodontics.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Kulzer Printer.
✅ Open Architecture
Or WhatsApp: +86 15951276160
