Introduction: Navigating the Global Market for dental implants vs dentures
In the evolving landscape of dental restoration, the choice between dental implants and dentures is a critical decision for healthcare providers and suppliers alike. As the demand for effective dental solutions continues to rise across Africa, South America, the Middle East, and Europe, understanding the nuances of these two options becomes essential for international B2B buyers. This guide offers an in-depth exploration of the types, materials, and manufacturing quality control associated with dental implants and dentures, along with insights into cost structures and market dynamics.
Navigating this complex market requires a keen awareness of the benefits and drawbacks of each solution. Dental implants, known for their durability and natural appearance, often represent a long-term investment in patient health. Conversely, dentures provide a more immediate and cost-effective option for those needing rapid restoration. This guide empowers buyers by presenting a comprehensive analysis of suppliers, enabling informed sourcing decisions that align with regional demands and regulatory considerations.
Whether you’re operating in Colombia, Germany, or elsewhere, this resource equips you with the knowledge to make strategic choices, ensuring that you can meet the diverse needs of your clientele while maximizing profitability. By understanding the implications of each option, you can enhance your offerings and contribute to improved patient outcomes in the global dental market.
Understanding dental implants vs dentures Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Complete Dentures | Replace all teeth in upper/lower jaw; removable | General dental practices, senior care | Pros: Cost-effective, non-invasive, easy to adjust. Cons: Less stable, can be uncomfortable, higher maintenance. |
| Partial Dentures | Replace some missing teeth; supported by existing teeth | Dental clinics, prosthodontics | Pros: Affordable, preserves remaining teeth. Cons: May shift, require frequent adjustments, less durable. |
| Traditional Implants | Titanium posts surgically placed in the jawbone | Oral surgery centers, specialty dental clinics | Pros: Long-lasting, feel like natural teeth. Cons: Higher initial cost, requires healthy bone structure. |
| All-on-4 Implants | Four implants support a full arch of prosthetic teeth | Full mouth restoration practices | Pros: Reduced surgery time, immediate function. Cons: Higher upfront cost, not suitable for all patients. |
| Mini Implants | Smaller diameter implants for limited bone structure | Cosmetic dentistry, implant specialty clinics | Pros: Less invasive, quicker recovery. Cons: May not be suitable for all applications, potentially less durable. |
Complete Dentures
Complete dentures are designed to replace all teeth in either the upper or lower jaw and are removable. They are primarily used in general dental practices and senior care facilities. When considering purchasing complete dentures, B2B buyers should note their affordability and non-invasive nature, making them an attractive option for patients. However, the potential for discomfort and the need for regular maintenance may deter some patients, impacting long-term satisfaction.
Partial Dentures
Partial dentures fill gaps when some natural teeth remain, utilizing clasps or precision attachments for support. They are commonly utilized in dental clinics and prosthodontics. B2B buyers should consider partial dentures for their cost-effectiveness and ability to preserve remaining teeth. However, the shifting nature and need for frequent adjustments can lead to dissatisfaction among patients, impacting repeat business for providers.
Traditional Implants
Traditional dental implants consist of titanium posts surgically inserted into the jawbone, providing a robust and lasting solution for missing teeth. They are ideal for oral surgery centers and specialty dental clinics. Buyers should recognize the long-term benefits of traditional implants, including their natural feel and durability. However, the higher initial costs and the necessity for a healthy jawbone may limit their accessibility for some patients, influencing purchasing decisions.
All-on-4 Implants
The All-on-4 implant technique uses four strategically placed implants to support an entire arch of prosthetic teeth. This method is favored in full mouth restoration practices. B2B buyers should appreciate the reduced surgery time and immediate function that All-on-4 implants offer. However, the higher upfront costs and specific patient eligibility requirements can be significant considerations when determining the marketability of this option.
Mini Implants
Mini implants are smaller diameter implants suitable for patients with limited jawbone structure. They are often used in cosmetic dentistry and implant specialty clinics. B2B buyers may find mini implants appealing due to their less invasive nature and quicker recovery times. However, their potential limitations in certain applications and comparatively lower durability should be carefully evaluated to ensure they meet patient needs effectively.
Related Video: Comparing All-on-4 vs Dental Implants vs Dentures
Key Industrial Applications of dental implants vs dentures
| Industry/Sector | Specific Application of dental implants vs dentures | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Dental Clinics | Providing restorative dental services | Increased patient satisfaction and retention | Quality of materials, supplier reliability, cost-effectiveness |
| Prosthetic Manufacturing | Production of custom dentures and implants | Enhanced product offerings and market competitiveness | Technological capabilities, customization options, lead times |
| Medical Equipment Distributors | Distribution of dental restoration products | Expanded product portfolio and revenue streams | Regulatory compliance, logistics capabilities, supplier relationships |
| Dental Laboratories | Fabrication of dental prosthetics | Improved turnaround times and service quality | Material sourcing, technology integration, pricing strategies |
| Health Insurance Companies | Coverage plans for dental restoration procedures | Increased customer loyalty and market differentiation | Understanding of local regulations, partnerships with dental providers |
Dental Clinics
Dental clinics serve as primary providers of restorative dental services, offering both dental implants and dentures to patients. Implants are preferred for their durability and natural feel, while dentures provide a more affordable option for those with budget constraints. Clinics must consider the quality of materials sourced, as well as the reliability of suppliers to ensure patient safety and satisfaction. Additionally, understanding the local market demand in regions like Africa or South America can help clinics tailor their offerings effectively.
Prosthetic Manufacturing
In the prosthetic manufacturing sector, companies focus on producing custom dentures and dental implants tailored to individual patient needs. The demand for high-quality, aesthetically pleasing products has led manufacturers to invest in advanced technologies and materials. This industry benefits from the ability to enhance product offerings, thus improving market competitiveness. Key considerations include the capability for customization, production lead times, and adherence to international standards, especially for buyers in Europe and the Middle East.
Medical Equipment Distributors
Medical equipment distributors play a crucial role in the supply chain for dental restoration products. By offering a range of implants and dentures, they can expand their product portfolio and create new revenue streams. Distributors must navigate the complexities of regulatory compliance, particularly when dealing with different international markets. Efficient logistics capabilities are also essential to ensure timely delivery of high-demand products, particularly in emerging markets in Africa and South America.
Dental Laboratories
Dental laboratories are integral in fabricating dental prosthetics, including both implants and dentures. They face the challenge of meeting tight turnaround times while maintaining high service quality. By investing in modern technologies and streamlining their processes, laboratories can enhance efficiency and customer satisfaction. Sourcing high-quality materials and integrating technology for better workflow management are vital considerations for B2B buyers in this sector.
Health Insurance Companies
Health insurance companies increasingly recognize the importance of offering coverage for dental restoration procedures, which can include both implants and dentures. By providing comprehensive coverage plans, insurers can enhance customer loyalty and differentiate themselves in a competitive market. Understanding local regulations and forming partnerships with dental providers are key strategies for these companies, especially in diverse markets such as Europe and the Middle East, where dental health awareness is growing.
Related Video: 3 Types of Dental Implants and Surface treatments explained!
Strategic Material Selection Guide for dental implants vs dentures
When selecting materials for dental implants and dentures, international B2B buyers must consider various factors, including material properties, manufacturing complexities, and compliance with local standards. Below is an analysis of four common materials used in these dental solutions.
Titanium for Dental Implants
Key Properties: Titanium is known for its excellent biocompatibility, corrosion resistance, and strength. It can withstand significant pressure and is resistant to temperature variations, making it ideal for oral applications.
Pros & Cons: Titanium implants are durable and can last a lifetime with proper care. However, they come with a higher manufacturing complexity and cost. The initial investment is significant, but the longevity and performance can justify the expense.
Impact on Application: Titanium is compatible with bone, promoting osseointegration, which is crucial for stability. This material is particularly effective in environments where bone density is a concern.
Considerations for International Buyers: Buyers from regions like Europe may prefer titanium due to stringent compliance with ASTM and ISO standards. In Africa and South America, the availability of titanium may affect pricing and accessibility, making it essential to consider local supply chains.
Acrylic Resin for Dentures
Key Properties: Acrylic resin is lightweight, easy to mold, and has good aesthetic qualities. It can be colored to match natural gum tissue, enhancing the appearance of dentures.
Pros & Cons: The primary advantage of acrylic resin is its affordability and ease of manufacturing. However, it is less durable than metals and may require replacement every 5-7 years. It is also prone to staining and may not withstand high temperatures well.
Impact on Application: Acrylic resin is suitable for creating both complete and partial dentures. Its flexibility allows for adjustments, making it easier to accommodate changes in the patient’s oral structure over time.
Considerations for International Buyers: Compliance with local standards such as DIN in Germany is crucial for acrylic resins. Buyers in the Middle East may need to consider the region’s climate, as extreme temperatures can affect the material’s performance.
Zirconia for Dental Implants
Key Properties: Zirconia is a ceramic material known for its strength, durability, and aesthetic appeal. It is also biocompatible and resistant to wear and corrosion.
Pros & Cons: Zirconia implants offer a natural appearance and are less likely to cause allergic reactions. However, they are more expensive than titanium and require precise manufacturing techniques, which can complicate production.
Impact on Application: Zirconia is ideal for patients seeking aesthetic solutions, as it mimics the look of natural teeth. It is suitable for environments where aesthetics are a priority, such as anterior teeth replacements.
Considerations for International Buyers: Buyers should be aware of the varying regulations regarding ceramic materials in different regions. In Europe, zirconia must meet high safety standards, while in Africa, sourcing may be limited, affecting availability and cost.
Cobalt-Chromium Alloy for Dentures
Key Properties: Cobalt-chromium alloys are known for their strength, resistance to corrosion, and ability to withstand high pressures. They are often used in frameworks for partial dentures.
Pros & Cons: The main advantage of cobalt-chromium is its durability and resistance to wear, making it suitable for long-term use. However, it is heavier than other materials and can be more expensive to manufacture.
Impact on Application: This alloy is particularly effective for patients with partial tooth loss, providing a robust framework that supports denture teeth. It is less likely to fracture under stress compared to acrylic.
Considerations for International Buyers: Compliance with JIS standards in Japan or ASTM in the U.S. is essential for cobalt-chromium alloys. Buyers in South America should consider local manufacturing capabilities, as importing these materials can increase costs.
Summary Table
| Material | Typical Use Case for dental implants vs dentures | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Titanium | Dental implants | Excellent biocompatibility and durability | High initial cost and manufacturing complexity | High |
| Acrylic Resin | Complete and partial dentures | Affordable and easy to adjust | Less durable, prone to staining | Low |
| Zirconia | Aesthetic dental implants | Natural appearance and biocompatibility | Higher cost and complex manufacturing | High |
| Cobalt-Chromium Alloy | Framework for partial dentures | Strong and corrosion-resistant | Heavier and more expensive to manufacture | Medium |
This analysis equips international B2B buyers with the necessary insights to make informed decisions regarding material selection for dental implants and dentures, ensuring compliance with local standards and meeting market demands.
In-depth Look: Manufacturing Processes and Quality Assurance for dental implants vs dentures
Understanding the manufacturing processes and quality assurance protocols for dental implants and dentures is essential for B2B buyers. This knowledge not only aids in making informed purchasing decisions but also ensures that the products meet the required safety and efficacy standards.
Manufacturing Processes
Dental Implants
-
Material Preparation
– Material Selection: Dental implants are primarily made from biocompatible materials, most commonly titanium or titanium alloys. These materials are chosen for their strength, durability, and ability to integrate with bone (osseointegration).
– Surface Treatment: The surface of the titanium is often treated (e.g., sandblasted or acid-etched) to enhance osseointegration. This step is critical as it affects the implant’s long-term stability. -
Forming
– CNC Machining: Computer Numerical Control (CNC) machining is used to create precise implant shapes. This high-precision technique ensures that the dimensions of the implants meet strict tolerances.
– Additive Manufacturing: Some manufacturers utilize 3D printing technologies to create custom implants or components, especially for complex cases. -
Assembly
– Component Integration: Implants often consist of multiple parts, including the fixture, abutment, and crown. These components must be assembled with high precision to ensure proper function.
– Sterilization: Post-assembly, implants undergo sterilization, typically through autoclaving or gamma radiation, to eliminate any microbial contamination before they reach the end user. -
Finishing
– Surface Finishing: Final surface treatments, such as anodization or coating with hydroxyapatite, are applied to enhance biocompatibility and promote osseointegration.
– Quality Inspection: Each batch of implants is subjected to rigorous inspection processes to ensure they meet the required specifications.
Dentures
-
Material Preparation
– Material Selection: Dentures are primarily made from acrylic resin, with some components made from metals (for partial dentures). The material must be both aesthetically pleasing and durable.
– Color Matching: The acrylic is often tinted to match the patient’s natural gum color, requiring careful preparation to ensure consistency. -
Forming
– Molding: Dentures are formed using molds created from impressions of the patient’s mouth. This step ensures a custom fit, critical for comfort and functionality.
– Heat Processing: The acrylic is heated and cured to achieve the desired hardness and shape. This process may involve both heat and pressure to ensure a uniform product. -
Assembly
– Teeth Placement: Artificial teeth are placed into the acrylic base, and adjustments are made to ensure proper alignment and occlusion.
– Finishing Touches: Dentures are polished to achieve a natural appearance and smooth edges, enhancing comfort for the wearer. -
Quality Inspection
– Final Inspection: Each denture is thoroughly inspected for fit, aesthetics, and structural integrity before being sent to the dentist or directly to the patient.
Quality Assurance
Quality assurance in the dental device manufacturing sector is governed by several international and industry-specific standards.
International Standards
- ISO 9001: This standard is crucial for ensuring overall quality management systems are in place. Manufacturers must demonstrate their ability to consistently provide products that meet customer and regulatory requirements.
- ISO 13485: Specifically tailored for medical devices, this standard outlines the requirements for a quality management system that demonstrates the manufacturer’s ability to provide medical devices and related services that consistently meet customer and regulatory requirements.
Industry-Specific Standards
- CE Marking: In Europe, products must meet certain safety, health, and environmental protection standards. The CE mark indicates compliance with European regulations.
- FDA Approval: In the United States, dental implants and dentures must be approved by the FDA, ensuring they are safe and effective for patient use.
Quality Control Checkpoints
-
Incoming Quality Control (IQC): This step involves the inspection of raw materials and components upon arrival at the manufacturing facility to ensure they meet specified criteria.
-
In-Process Quality Control (IPQC): During manufacturing, various checkpoints are established to monitor the production process. This includes dimensional checks and material property evaluations.
-
Final Quality Control (FQC): Before products are released, a comprehensive inspection is conducted to verify that they meet all specifications and standards. This includes functional testing and visual inspections.
Common Testing Methods
- Mechanical Testing: Ensures that implants can withstand the forces they will encounter in the oral environment.
- Biocompatibility Testing: Assesses the safety of materials used in dental devices to prevent adverse reactions in patients.
- Sterility Testing: Confirms that products are free from viable microorganisms, a critical requirement for implants and dentures.
Verifying Supplier Quality Control
B2B buyers should adopt a systematic approach to verify the quality control processes of their suppliers:
- Supplier Audits: Conduct regular audits of manufacturing facilities to assess compliance with international standards and internal quality processes.
- Quality Reports: Request detailed quality assurance reports that outline testing methods, results, and any corrective actions taken.
- Third-Party Inspections: Engage independent third-party organizations to conduct inspections and testing to ensure unbiased evaluation of product quality.
QC and Certification Nuances for International Buyers
For international buyers, particularly from regions like Africa, South America, the Middle East, and Europe, understanding regional certifications and compliance can be complex. Here are some key considerations:
- Local Regulations: Familiarize yourself with local regulations that may affect the importation and sale of dental devices in your country.
- Certification Recognition: Ensure that the certifications held by your suppliers (e.g., CE, FDA) are recognized and accepted in your local market.
- Cultural Considerations: Be aware of cultural preferences and requirements that may influence the design and functionality of dental products in different regions.
By understanding the manufacturing processes and quality assurance protocols for dental implants and dentures, B2B buyers can make informed decisions that enhance patient care and maintain high standards in dental health.
Comprehensive Cost and Pricing Analysis for dental implants vs dentures Sourcing
When evaluating the cost structure for dental implants and dentures, it is essential to break down the various components that contribute to the overall pricing. Understanding these factors will enable B2B buyers to make informed decisions and optimize their sourcing strategies.
Cost Components
- Materials: The raw materials used significantly influence the cost. Dental implants typically utilize high-grade titanium for biocompatibility and strength, while dentures often consist of acrylic resins and other polymers. The quality of materials can vary widely, affecting both price and durability.
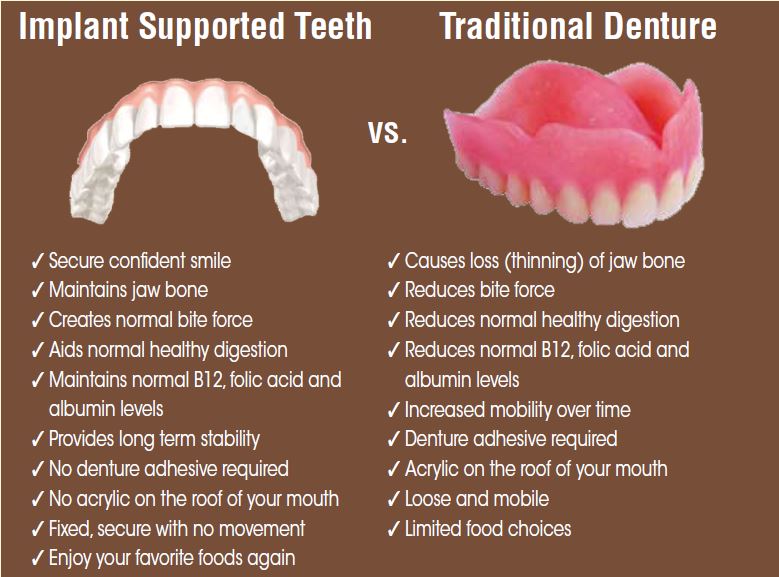
Illustrative Image (Source: Google Search)
-
Labor: Labor costs can vary based on the complexity of the product. Implants require skilled professionals for surgical placement and may involve multiple specialists (e.g., oral surgeons, prosthodontists), leading to higher labor costs. In contrast, dentures involve less invasive procedures, but still require skilled technicians for fitting and adjustments.
-
Manufacturing Overhead: This includes costs associated with maintaining production facilities and equipment. Dental implant manufacturing generally requires more advanced technology and quality control measures, contributing to higher overhead costs compared to dentures.
-
Tooling: The design and production of custom implants necessitate specialized tooling, which can be a significant upfront investment. Conversely, dentures often use standardized molds, reducing tooling costs.
-
Quality Control (QC): Implants undergo stringent QC processes due to their surgical application and long-term use. This adds to their cost, while dentures may have less rigorous QC standards, impacting their pricing.
-
Logistics: Transportation costs vary based on the destination and the nature of the product. Implants may require more careful handling and expedited shipping, while dentures are typically less sensitive to logistics variations.
-
Margin: The profit margin can differ significantly between products. Manufacturers of dental implants may command higher margins due to the perceived value and longevity of the product, while dentures generally have lower margins due to their lower price point.
Price Influencers
-
Volume/MOQ: Bulk purchasing often leads to discounts. Buyers should consider negotiating minimum order quantities to lower per-unit costs.
-
Specifications/Customization: Custom implants tailored to specific patient needs can increase costs significantly. Standardized dentures can be produced at lower prices, making them more accessible.
-
Materials: The choice between premium and standard materials will directly affect pricing. Buyers should assess the trade-offs between cost and quality.
-
Quality/Certifications: Products with higher certifications (ISO, CE marking) may have increased costs but offer better assurance of quality and safety, especially important in healthcare.
-
Supplier Factors: The reputation and reliability of suppliers can influence costs. Established suppliers may charge more for their products but often provide better support and guarantees.
-
Incoterms: Understanding shipping terms is crucial for international buyers. Costs can vary significantly based on the chosen Incoterm, affecting total landed costs.
Buyer Tips
-
Negotiation: Engage in discussions with suppliers to explore discounts based on volume or long-term contracts. Establishing a good relationship can also lead to better terms.
-
Cost-Efficiency: Evaluate the total cost of ownership, not just the purchase price. Consider factors like maintenance, longevity, and potential need for replacements.
-
Pricing Nuances for International Buyers: Be aware of currency fluctuations, tariffs, and import duties when sourcing internationally. Local regulations and market conditions in regions like Africa, South America, the Middle East, and Europe can also impact pricing strategies.
Disclaimer
The prices for dental implants and dentures can vary widely based on the factors mentioned above. The figures provided in this analysis are indicative and should be confirmed with suppliers for accurate quotes and terms.
Spotlight on Potential dental implants vs dentures Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘dental implants vs dentures’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for dental implants vs dentures
When evaluating dental implants and dentures, understanding the essential technical properties and trade terminology is crucial for making informed purchasing decisions. Below are key specifications and terms that will aid B2B buyers in navigating the complexities of these dental restoration options.
Key Technical Properties
-
Material Composition
– Dental Implants: Typically made from titanium or zirconia, these materials are chosen for their biocompatibility and strength. Titanium integrates with the jawbone through a process called osseointegration, which is vital for the stability of the implant.
– Dentures: Usually crafted from acrylic resin or a combination of acrylic and metal frameworks. The choice of material affects durability, aesthetic appeal, and comfort. Understanding the material can help buyers assess the longevity and maintenance requirements of dentures. -
Fit and Tolerance
– The precision of fit is critical for both implants and dentures. For implants, the tolerance between the implant and the bone must be minimal to ensure stability. For dentures, a snug fit is necessary to prevent slippage and discomfort during use.
– Accurate fit specifications help in reducing the need for adjustments and enhancing patient satisfaction. -
Longevity and Durability
– Dental Implants: Generally last a lifetime with proper care, making them a cost-effective long-term solution. They require a higher initial investment but offer better value over time.
– Dentures: Typically last between 5 to 10 years, necessitating replacements or adjustments due to wear or changes in the jaw structure. Understanding these timelines aids buyers in budget forecasting. -
Aesthetic Customization
– Customization options, including tooth shade, shape, and gum color, are vital for ensuring natural appearance. For implants, the crown can be tailored to match existing teeth, while dentures can be designed to restore the patient’s facial aesthetics.
– Aesthetic considerations are crucial for patient satisfaction and can influence purchasing decisions. -
Maintenance Requirements
– Dental implants require standard oral hygiene practices, similar to natural teeth. In contrast, dentures must be removed for cleaning and require special adhesives.
– Understanding maintenance needs can impact long-term patient compliance and satisfaction, affecting product selection.
Common Trade Terminology
-
OEM (Original Equipment Manufacturer)
– Refers to companies that produce parts or equipment that may be marketed by another manufacturer. In the dental industry, OEMs provide implants and denture components that meet specific standards and specifications. -
MOQ (Minimum Order Quantity)
– The smallest amount of a product that a supplier is willing to sell. For B2B buyers, understanding MOQ is essential for budgeting and inventory management, especially when sourcing dental products. -
RFQ (Request for Quotation)
– A document issued by a buyer to solicit price bids from suppliers. An RFQ is crucial for comparing costs and ensuring that the buyer receives competitive pricing for dental implants or dentures. -
Incoterms (International Commercial Terms)
– A set of international rules that define the responsibilities of sellers and buyers in international transactions. Understanding Incoterms is vital for B2B buyers to clarify shipping costs, risks, and responsibilities when importing dental products from other regions. -
Regulatory Compliance
– Refers to adherence to laws and regulations governing the manufacture and sale of dental products. For buyers, ensuring that products meet local and international standards is critical for patient safety and legal compliance. -
Warranty and Service Agreements
– These are commitments from manufacturers regarding the quality and durability of their products. A clear understanding of warranty terms can help B2B buyers mitigate risks associated with product failures or defects.
By familiarizing themselves with these technical properties and trade terminologies, B2B buyers can enhance their decision-making processes when selecting dental implants or dentures, ultimately leading to better patient outcomes and satisfaction.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the dental implants vs dentures Sector
Global drivers influencing the dental implants and dentures market include an aging population, increasing awareness of oral health, and advancements in dental technology. Countries in Africa, South America, the Middle East, and Europe are witnessing a growing demand for both dental implants and dentures, driven by rising disposable incomes and an expanding middle class. In regions like Germany, there is a notable preference for dental implants due to their long-term benefits and functionality, while in Colombia, affordability and accessibility often lead to a higher adoption of dentures.
Emerging trends in B2B sourcing reflect a shift towards digital solutions, with an increasing reliance on online platforms for procurement and supply chain management. Companies are leveraging technology to streamline operations, enhance customer engagement, and improve the overall purchasing experience. Additionally, the use of 3D printing technology is gaining traction, allowing for customized dental solutions that cater to individual patient needs. This trend is particularly relevant for international buyers who are looking for innovative, cost-effective solutions that can be tailored to their markets.
Market dynamics also highlight the importance of regulatory compliance and quality assurance. International buyers must navigate varying regulatory landscapes, ensuring that products meet local health and safety standards. As competition intensifies, companies that prioritize innovation and customer-centric approaches will be better positioned to capture market share.
Sustainability & Ethical Sourcing in B2B
Sustainability has become a critical consideration in the dental implants and dentures sector. The production of dental materials can have significant environmental impacts, including waste generation and resource depletion. As such, buyers are increasingly focused on sourcing materials that minimize environmental harm. This includes the use of biocompatible and recyclable materials in dental implants and dentures, which not only reduces waste but also aligns with growing consumer demand for eco-friendly products.
Ethical supply chains are becoming paramount in this industry. International buyers must ensure that their suppliers adhere to responsible labor practices and sustainable sourcing methods. This is particularly crucial in regions where regulatory oversight may be less stringent. Certifications such as ISO 14001 for environmental management and FSC certification for sustainable forestry can guide buyers in making responsible purchasing decisions.
Moreover, transparency in the supply chain is essential. Buyers should seek manufacturers that provide clear information about their sourcing practices, allowing for informed decisions that support sustainability and ethical standards.
Brief Evolution/History
The evolution of dental implants and dentures has been shaped by advancements in dental science and technology. Historically, dentures have been used for centuries as a solution for tooth loss, evolving from crude materials to more sophisticated designs that mimic natural aesthetics and function.
Dental implants, on the other hand, are a more recent innovation, gaining prominence since the 1960s when titanium was introduced as a biocompatible material. This breakthrough allowed for the development of durable, long-lasting implants that closely resemble natural teeth. The shift towards implants reflects broader trends in dentistry favoring more permanent, effective solutions over temporary fixes like dentures. As both markets continue to evolve, B2B buyers must remain vigilant to emerging technologies and consumer preferences that shape their sourcing strategies.
Frequently Asked Questions (FAQs) for B2B Buyers of dental implants vs dentures
-
What key factors should I consider when vetting suppliers for dental implants and dentures?
When vetting suppliers, prioritize their certifications, such as ISO and CE marks, which indicate compliance with international quality standards. Investigate their production capacity, lead times, and experience in the industry. Additionally, assess their customer service responsiveness and willingness to provide product samples. It’s also wise to seek reviews from other B2B buyers and verify the supplier’s reputation through trade associations or industry networks relevant to your region. -
Can dental implants and dentures be customized to meet specific regional demands?
Yes, many suppliers offer customization options to cater to local aesthetic preferences, cultural practices, and specific clinical requirements. Customization can include variations in size, shape, and material used for both implants and dentures. Engaging in dialogue with suppliers about your specific needs can lead to tailored solutions that enhance patient satisfaction and market competitiveness. -
What are the typical minimum order quantities (MOQs) and lead times for dental implants and dentures?
MOQs can vary widely based on the supplier and the type of product. For dental implants, MOQs might range from 50 to 100 units, while dentures could have lower MOQs, especially if they are pre-manufactured. Lead times also differ, typically spanning from a few weeks to several months, depending on customization and production schedules. Always confirm these details during initial negotiations to plan your inventory effectively. -
What payment terms are commonly offered by suppliers of dental implants and dentures?
Payment terms can vary, but many suppliers require a deposit upfront, with the remaining balance due upon delivery or before shipping. Some may offer credit terms, allowing payment within 30 to 90 days post-delivery. It’s essential to negotiate favorable terms that align with your cash flow requirements while ensuring supplier reliability and quality assurance. -
How can I ensure quality assurance and certification for dental products?
Request documentation of quality assurance processes, including certificates of compliance and testing results. Reputable suppliers will provide evidence of their adherence to international quality standards. Additionally, consider third-party audits or certifications that can validate the supplier’s claims. Establishing a quality control protocol for incoming products can further safeguard against defects. -
What logistics considerations should I keep in mind when sourcing dental implants and dentures internationally?
International logistics can be complex, so consider customs regulations, shipping costs, and potential delays. Ensure that your suppliers are familiar with export documentation and can assist with logistics planning. It’s also beneficial to work with a logistics partner experienced in handling medical devices to navigate any specific import/export requirements in your target markets. -
What steps should I take if I encounter disputes with suppliers?
Establish clear communication channels and maintain detailed records of all transactions to facilitate dispute resolution. In case of a disagreement, attempt to resolve the issue amicably through direct negotiation. If necessary, refer to the terms outlined in your contract regarding dispute resolution mechanisms, such as mediation or arbitration. Having a legal advisor familiar with international trade can also be beneficial in these situations. -
What are the emerging trends in dental implants and dentures that I should be aware of?
Stay informed about advancements in materials, such as biocompatible options for implants and 3D printing technologies for dentures, which can enhance customization and reduce production times. Additionally, the trend towards digital dentistry, including virtual consultations and digital impressions, is gaining traction, improving patient experience and outcomes. Keeping abreast of these trends can help you align your offerings with market demands and position your business competitively.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for dental implants vs dentures
In navigating the complex landscape of dental restoration options, strategic sourcing plays a pivotal role for international B2B buyers. Dental implants provide a long-term solution with superior functionality and aesthetics, making them an attractive investment despite their higher upfront costs. Conversely, dentures offer an immediate, more affordable alternative, though they may require more frequent replacements and maintenance.
For buyers in regions such as Africa, South America, the Middle East, and Europe, understanding the nuances of these products is essential. Prioritizing quality suppliers who can deliver both implants and dentures tailored to local market needs can enhance patient satisfaction and foster long-term relationships. Furthermore, leveraging local partnerships may reduce logistical challenges and improve cost efficiency.
Illustrative Image (Source: Google Search)
As the dental market continues to evolve, staying informed about technological advancements and patient preferences will be crucial. Embrace the opportunity to engage with innovative manufacturers and distributors, ensuring your offerings remain competitive. Act now to position your business at the forefront of this growing market, and make informed decisions that will shape the future of dental care in your region.
