Introduction: Navigating the Global Market for dental implant adhesive
In the rapidly evolving landscape of dental healthcare, the role of dental implant adhesive cannot be overstated. This critical component serves not only to secure dental implants but also to enhance the longevity and effectiveness of restorative procedures. For international B2B buyers, particularly those operating in diverse markets such as Africa, South America, the Middle East, and Europe, understanding the nuances of dental implant adhesive is essential for making informed sourcing decisions.
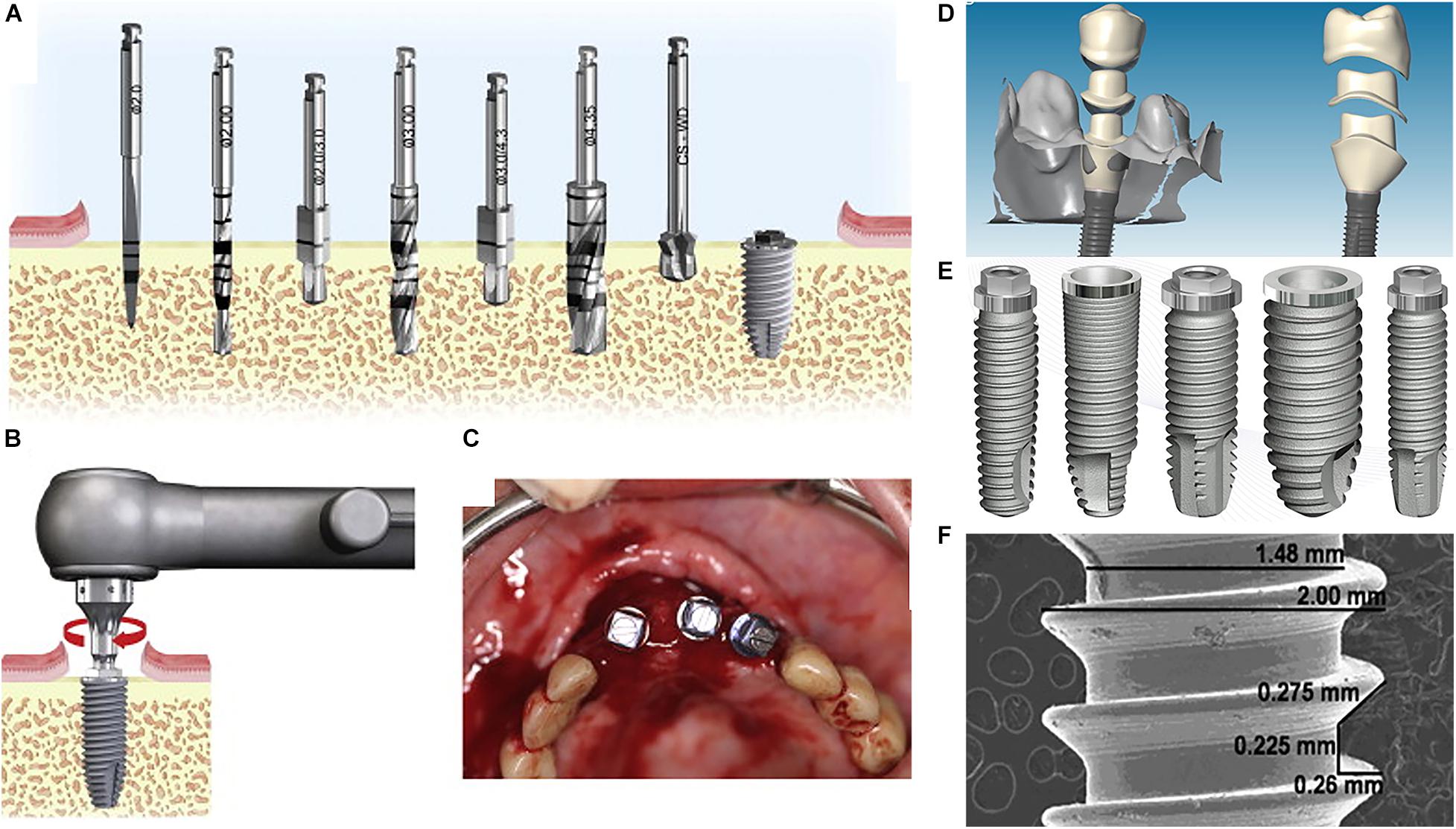
Illustrative Image (Source: Google Search)
This guide offers a comprehensive overview of dental implant adhesives, delving into various types and materials available in the market. We will explore manufacturing processes and quality control measures that ensure the reliability and performance of these products. Additionally, we will highlight key suppliers and their offerings, enabling buyers to identify reputable sources that align with their needs.
Cost considerations are also pivotal; understanding pricing structures and factors influencing costs will empower buyers to negotiate effectively and maximize value. To further enhance decision-making, we will address frequently asked questions that clarify common concerns and misconceptions surrounding dental implant adhesives.
By equipping B2B buyers with in-depth knowledge and actionable insights, this guide aims to facilitate strategic sourcing and foster successful partnerships in the dental implant sector. Whether you are in Spain or Kenya, navigating the global market for dental implant adhesive has never been more accessible or critical to your business success.
Understanding dental implant adhesive Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Cement-based Adhesives | Strong bonding, easy application | Dental clinics, laboratories | Pros: Reliable bond; Cons: Longer curing time |
| Resin-based Adhesives | High strength, moisture resistant | High-end dental practices | Pros: Quick setting; Cons: Higher cost |
| Glass Ionomer Adhesives | Fluoride release, biocompatible | Pediatric dentistry, general use | Pros: Good for pediatric use; Cons: Lower strength |
| Epoxy Adhesives | Excellent chemical resistance | Specialty dental applications | Pros: Durable; Cons: Complex application process |
| Self-adhesive Systems | Simplified application, no mixing required | General dental practices | Pros: User-friendly; Cons: Variable strength |
Cement-based Adhesives
Cement-based adhesives are widely recognized for their strong bonding capabilities and ease of application. These adhesives are particularly suitable for use in dental clinics and laboratories where reliability is paramount. B2B buyers should consider the curing time, as these adhesives typically require longer setting periods, which may impact workflow in high-volume practices. However, their proven effectiveness makes them a staple in many dental applications.
Resin-based Adhesives
Resin-based adhesives are favored for their high strength and moisture resistance, making them ideal for high-end dental practices that demand superior performance. They set quickly, allowing for faster patient turnover, which is essential in busy clinics. However, B2B buyers should be mindful of the higher cost associated with these adhesives, as they may impact overall budget considerations. Their efficiency can justify the expense in competitive markets.
Glass Ionomer Adhesives
Glass ionomer adhesives are known for their unique properties, including fluoride release and biocompatibility, making them particularly suitable for pediatric dentistry and general use. Their ability to bond chemically with dental tissues offers a distinct advantage in certain applications. Buyers should weigh the lower strength of these adhesives against their benefits, especially in scenarios where patient safety and comfort are paramount. They represent a good option for practices focusing on young patients.
Epoxy Adhesives
Epoxy adhesives are characterized by their excellent chemical resistance and durability, making them suitable for specialty dental applications where extreme conditions may be encountered. B2B buyers should consider the complexity of the application process, as these adhesives often require precise mixing and application techniques. While they offer robust performance, the learning curve and potential for application errors may deter some practices from utilizing them.
Self-adhesive Systems
Self-adhesive systems simplify the bonding process by eliminating the need for mixing, making them user-friendly for general dental practices. These adhesives are particularly appealing to practices looking to streamline operations and reduce training time for staff. However, buyers should be aware of the variability in strength compared to other adhesive types. Understanding the specific needs of their practice will help buyers determine if the convenience outweighs potential performance trade-offs.
Related Video: Dental Implant Overdenture | Snap-In Dentures
Key Industrial Applications of dental implant adhesive
| Industry/Sector | Specific Application of dental implant adhesive | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Dental Clinics | Securing dental implants in restorative procedures | Enhances patient satisfaction and retention | Ensure compliance with local health regulations and standards. |
| Dental Laboratories | Fabricating dental prosthetics and fixtures | Improves accuracy and durability of dental products | Source adhesives with rapid curing times to streamline production. |
| Orthopedic Manufacturing | Adhesives for bone implants and orthopedic devices | Expands product offerings and market reach | Look for biocompatible adhesives that meet international safety standards. |
| Research Institutions | Development of dental materials and testing | Supports innovation and research capabilities | Collaborate with suppliers who provide technical support and customization options. |
| Veterinary Clinics | Application in animal dental procedures | Addresses a niche market and enhances service offerings | Consider adhesives that are safe for animal use and comply with veterinary regulations. |
Dental Clinics
In dental clinics, dental implant adhesive is essential for securing implants during restorative procedures. This adhesive ensures that the implants remain stable, improving the longevity of the dental work and enhancing patient satisfaction. For international B2B buyers, particularly in regions like Africa and South America, it is crucial to select adhesives that comply with local health regulations, ensuring safety and efficacy in clinical applications.
Dental Laboratories
Dental laboratories utilize dental implant adhesive for fabricating prosthetics and fixtures. The adhesive plays a critical role in ensuring the accuracy and durability of dental products, which can significantly impact patient outcomes. Buyers in Europe and the Middle East should prioritize sourcing adhesives with rapid curing times to streamline production processes and meet the high demand for quality dental solutions.
Orthopedic Manufacturing
In the orthopedic manufacturing sector, dental implant adhesive is increasingly being adapted for use in bone implants and other orthopedic devices. This application allows manufacturers to expand their product offerings into a lucrative market while ensuring the safety and effectiveness of their products. International buyers must focus on sourcing biocompatible adhesives that meet stringent international safety standards, particularly in regions with rigorous regulatory frameworks.
Research Institutions
Research institutions leverage dental implant adhesives for the development and testing of new dental materials. This application supports innovation and enhances research capabilities, allowing institutions to stay at the forefront of dental technology advancements. B2B buyers should seek suppliers who provide not only high-quality adhesives but also technical support and customization options to facilitate specific research needs.
Veterinary Clinics
Veterinary clinics are beginning to adopt dental implant adhesive for use in animal dental procedures. This application addresses a niche market, allowing clinics to enhance their service offerings and cater to pet owners seeking advanced dental care for their animals. Buyers in this sector should prioritize adhesives that are safe for animal use and ensure compliance with veterinary regulations to maintain ethical standards in practice.
Related Video: NobelActive clinical case: immediate implant placement – Eric Rompen
Strategic Material Selection Guide for dental implant adhesive
When selecting materials for dental implant adhesives, international B2B buyers must consider various factors that impact performance, cost, and compliance with regional standards. Here, we analyze four common materials used in dental implant adhesives, focusing on their properties, advantages, disadvantages, and specific considerations for buyers from Africa, South America, the Middle East, and Europe.
Epoxy Resins
Key Properties:
Epoxy resins are known for their excellent adhesion, chemical resistance, and durability. They can withstand high temperatures and pressures, making them suitable for various dental applications.
Pros & Cons:
Epoxy adhesives offer exceptional strength and durability, ensuring long-lasting bonds. However, they can be more expensive than other options, and their manufacturing process may be complex, requiring precise mixing and curing conditions.
Impact on Application:
These adhesives are compatible with a wide range of dental materials, including metals and ceramics, which is crucial for dental implants.
Considerations for International Buyers:
Buyers should ensure that the epoxy resins comply with relevant standards such as ASTM D-1002 or ISO 10993 for biocompatibility. Additionally, understanding the local regulations regarding chemical use is essential, particularly in regions with stringent environmental laws.
Polyurethane Adhesives
Key Properties:
Polyurethane adhesives are versatile and exhibit excellent flexibility and impact resistance. They can bond well to a variety of substrates, including metals and plastics.
Pros & Cons:
The flexibility of polyurethane adhesives allows for movement between bonded surfaces, making them suitable for applications where slight shifts may occur. However, they may have lower temperature and chemical resistance compared to epoxy resins, which could limit their use in certain environments.
Impact on Application:
These adhesives are particularly effective in applications where shock absorption is necessary, such as in dental implants subjected to dynamic forces.
Considerations for International Buyers:
Buyers should verify that polyurethane adhesives meet standards like DIN 55733 or JIS K 6850. Additionally, considering the climate of the region (e.g., high humidity in parts of Africa and South America) is vital, as it can affect adhesive performance.
Cyanoacrylate Adhesives
Key Properties:
Cyanoacrylate adhesives, commonly known as “super glues,” cure rapidly and provide strong bonds. They are resistant to moisture and can bond to various materials quickly.
Pros & Cons:
The rapid curing time is a significant advantage, allowing for quick assembly and reduced production time. However, cyanoacrylate adhesives are generally less durable under stress and may not be suitable for high-load applications.
Impact on Application:
These adhesives work well in temporary applications or where quick bonding is essential, but their limited durability may restrict their use in permanent dental implant scenarios.
Considerations for International Buyers:
Buyers should ensure compliance with ISO 10993 for medical applications and consider the availability of cyanoacrylate adhesives in their local markets, as sourcing can vary significantly by region.
Silicone Adhesives
Key Properties:
Silicone adhesives are known for their flexibility, temperature resistance, and excellent weathering properties. They can maintain adhesion in varying environmental conditions.
Pros & Cons:
Silicone adhesives are excellent for applications requiring flexibility and resistance to environmental factors. However, they may not provide the same level of strength as epoxy or polyurethane adhesives, which could be a limitation in load-bearing applications.
Impact on Application:
Silicone adhesives are particularly suitable for applications where expansion and contraction are expected, making them ideal for certain dental implant configurations.
Considerations for International Buyers:
Buyers should check for compliance with ASTM C-920 or similar standards and consider the specific environmental conditions of their regions, such as temperature fluctuations and humidity levels.
Summary Table
| Material | Typical Use Case for dental implant adhesive | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Epoxy Resins | Permanent bonding of dental implants | Exceptional strength and durability | Higher cost and complex manufacturing | High |
| Polyurethane Adhesives | Shock-absorbing applications | Flexibility and impact resistance | Lower temperature resistance | Medium |
| Cyanoacrylate Adhesives | Temporary bonding or quick assembly | Rapid curing time | Limited durability under stress | Low |
| Silicone Adhesives | Flexible applications | Excellent weathering properties | Lower strength compared to others | Medium |
This guide offers a strategic overview of material selection for dental implant adhesives, enabling international B2B buyers to make informed decisions that align with their operational needs and regional standards.
In-depth Look: Manufacturing Processes and Quality Assurance for dental implant adhesive
Manufacturing dental implant adhesives involves a series of meticulously controlled processes to ensure the final product meets the stringent requirements of safety, efficacy, and performance. For international B2B buyers, particularly from regions such as Africa, South America, the Middle East, and Europe, understanding these processes is crucial for making informed purchasing decisions.
Key Stages in the Manufacturing Process
-
Material Preparation
– Selection of Raw Materials: The choice of raw materials is fundamental. Common components include methacrylate monomers, initiators, and fillers. B2B buyers should inquire about the sourcing of these materials, as quality can significantly impact the adhesive’s performance.
– Chemical Mixing: Precise formulations are developed using advanced mixing techniques to ensure uniform distribution of components. This step often employs high-shear mixing equipment to achieve a homogeneous blend, which is critical for the adhesive’s final properties. -
Forming
– Casting and Molding: Once mixed, the adhesive is cast into molds or formed into specific shapes (e.g., syringes or vials). This step requires careful control of temperature and pressure to avoid any alterations in the adhesive’s characteristics.
– Curing Process: The adhesive undergoes curing, typically through heat or UV light, to achieve the desired physical and chemical properties. Buyers should ask about the curing methods used, as they can affect the adhesive’s strength and biocompatibility. -
Assembly
– Packaging: After curing, the adhesive is packaged in sterile conditions to prevent contamination. The packaging must maintain the adhesive’s integrity and ensure ease of use for dental professionals.
– Labeling: Proper labeling is essential for compliance with international standards, providing information on usage, storage conditions, and expiration dates. -
Finishing
– Quality Control: At this stage, the products undergo rigorous quality control checks to ensure they meet specifications. This includes visual inspections and dimensional checks.
– Sterilization: Many dental adhesives require sterilization to eliminate any microbial contamination. Buyers should verify the sterilization methods employed, as this is crucial for patient safety.
Quality Assurance Processes
Quality assurance (QA) is an integral part of the manufacturing process, ensuring that dental implant adhesives meet international and industry-specific standards.
Relevant International Standards
- ISO 9001: This standard outlines the requirements for a quality management system (QMS) and is crucial for manufacturers aiming to ensure consistent quality and customer satisfaction.
- CE Marking: For products marketed in Europe, CE marking signifies compliance with EU safety, health, and environmental protection standards.
- API Standards: In some cases, adherence to the American Petroleum Institute (API) standards may be relevant, especially if the adhesive components include petroleum-derived substances.
Quality Control Checkpoints
- Incoming Quality Control (IQC): This involves inspecting raw materials upon receipt to ensure they meet specifications before production begins. B2B buyers should request IQC reports to understand the quality of materials used.
- In-Process Quality Control (IPQC): During manufacturing, ongoing checks are conducted to monitor processes and detect any deviations from standards. This includes monitoring temperature, humidity, and equipment performance.
- Final Quality Control (FQC): After production, finished products undergo thorough testing, including physical and chemical tests, to confirm they meet all regulatory and performance standards.
Common Testing Methods
- Viscosity Testing: Measures the adhesive’s flow properties, which can affect application ease and bonding strength.
- Tensile Strength Testing: Assesses the adhesive’s ability to withstand forces without breaking, critical for ensuring durability in dental applications.
- Biocompatibility Testing: Ensures that the adhesive is safe for use in the human body, particularly important for dental applications.
Verifying Supplier Quality Control
For B2B buyers, particularly those sourcing from international suppliers, verifying the quality control processes of potential partners is essential. Here are some actionable insights:
- Conduct Audits: Regular audits of suppliers can reveal insights into their manufacturing practices and adherence to quality standards. This can be done either by the purchasing company or through third-party audit services.
- Request Quality Reports: Suppliers should provide detailed quality reports, including IQC, IPQC, and FQC findings, which can help buyers assess the reliability of the manufacturing process.
- Third-Party Inspections: Engaging independent third-party inspection services can provide an unbiased assessment of the supplier’s quality control measures and compliance with international standards.
Navigating QC and Certification Nuances
International buyers, especially from diverse regions like Africa, South America, the Middle East, and Europe, should be aware of the varying requirements for quality control and certifications:
- Regional Regulations: Different regions may have unique regulations that impact the certification process. For instance, the FDA in the U.S. has different requirements than the EMA in Europe.
- Cultural Considerations: Understanding cultural perspectives on quality and safety can influence negotiations and partnerships. Buyers should engage in discussions with suppliers about their quality philosophies and practices.
- Building Relationships: Establishing strong relationships with suppliers can facilitate better transparency regarding quality control practices and enable easier resolution of potential quality issues.
By gaining insights into the manufacturing processes and quality assurance protocols for dental implant adhesives, international B2B buyers can make informed choices that align with their quality expectations and regulatory requirements.
Comprehensive Cost and Pricing Analysis for dental implant adhesive Sourcing
Analyzing the cost structure and pricing dynamics of dental implant adhesives is crucial for international B2B buyers looking to optimize their sourcing strategies. This section outlines the primary cost components, price influencers, and actionable tips tailored for buyers from regions such as Africa, South America, the Middle East, and Europe.
Cost Components
-
Materials: The primary cost driver in dental implant adhesives is the raw materials used, including polymers and bonding agents. Prices can fluctuate based on market demand and availability of these materials, impacting overall costs.
-
Labor: Labor costs vary significantly depending on the production location. Regions with lower labor costs may offer competitive pricing, but this can also affect quality. Buyers should assess labor conditions to ensure that quality standards are met.
-
Manufacturing Overhead: This includes utilities, equipment maintenance, and general operational expenses. Efficient manufacturing processes can help reduce overhead costs, which can be passed on to buyers.
-
Tooling: Investment in specialized tools and molds for production is essential, particularly for custom adhesive formulations. The cost of tooling can be amortized over larger production runs, making it crucial to consider Minimum Order Quantities (MOQs).
-
Quality Control (QC): Implementing rigorous QC processes ensures product reliability but adds to the cost. Buyers should seek suppliers who maintain high standards and certifications to mitigate risks associated with product failure.
-
Logistics: Shipping, handling, and customs duties can significantly impact the total cost of sourcing. The choice of Incoterms can influence how these costs are managed between the buyer and supplier.
-
Margin: Suppliers typically add a margin to cover their costs and profit. Understanding the typical margins in the industry can help buyers negotiate better deals.
Price Influencers
Several factors can influence the pricing of dental implant adhesives:
-
Volume/MOQ: Higher order volumes often lead to lower per-unit costs. Buyers should consider consolidating orders to leverage better pricing.
-
Specifications/Customization: Customized products may incur additional costs. Buyers should clarify their needs upfront to avoid unexpected expenses.
-
Materials: The choice of materials affects both performance and cost. High-quality materials may come at a premium but can lead to better patient outcomes and reduced liability.
-
Quality/Certifications: Products that meet international standards (e.g., ISO, CE) may be priced higher but provide assurance of quality and safety.
-
Supplier Factors: The reputation and reliability of suppliers can impact pricing. Established suppliers may charge more but offer greater assurance of product consistency and support.
-
Incoterms: Understanding the implications of different shipping terms is essential. Some terms may shift responsibilities and costs, impacting the overall price.
Buyer Tips
-
Negotiation: Leverage your purchasing power by negotiating terms, especially if placing bulk orders. It’s beneficial to build long-term relationships with suppliers for better pricing over time.
-
Cost-Efficiency: Focus on the Total Cost of Ownership (TCO), which includes not just the purchase price but also logistics, storage, and potential product failures.
-
Pricing Nuances: International buyers should be aware of currency fluctuations, tariffs, and local taxes that may affect the final cost. Establishing contracts in stable currencies can mitigate risks.
-
Market Research: Stay informed about market trends and material prices. This knowledge can empower buyers during negotiations and help identify the right time to purchase.
-
Supplier Audits: Conducting audits or reviews of potential suppliers can provide insights into their operational efficiencies and cost structures, helping buyers make informed decisions.
Disclaimer
The prices and costs discussed in this section are indicative and subject to change based on market conditions, supplier negotiations, and specific buyer requirements. Always conduct thorough due diligence and request detailed quotes from multiple suppliers to ensure competitive pricing.
Spotlight on Potential dental implant adhesive Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘dental implant adhesive’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for dental implant adhesive
Key Technical Properties of Dental Implant Adhesives
When selecting dental implant adhesives, understanding their technical properties is crucial for ensuring compatibility, performance, and regulatory compliance. Below are some essential specifications that B2B buyers should consider:
-
Material Composition: Dental implant adhesives are typically composed of polymers, such as cyanoacrylate or epoxy resins. The choice of material affects the adhesive’s bonding strength, curing time, and biocompatibility. For instance, cyanoacrylate adhesives are known for their rapid curing and strong bond, making them suitable for immediate dental applications.
-
Viscosity: This property indicates how thick or thin the adhesive is, which affects its application and penetration into small gaps between the implant and bone or tissue. Lower viscosity adhesives may flow better, ensuring an even distribution, while higher viscosity options can provide better control during application. Understanding viscosity is essential for selecting adhesives that match specific procedural needs.
-
Cure Time: The time it takes for the adhesive to fully set is a critical factor in dental procedures. A shorter cure time can be beneficial in surgeries where time is of the essence, while longer cure times may allow for adjustments before the adhesive sets. Buyers should consider the implications of cure time on workflow efficiency.
-
Shear Strength: This measurement indicates the adhesive’s ability to withstand forces that can cause sliding between bonded surfaces. High shear strength is crucial for dental implants as they are subject to significant forces during chewing. Buyers should seek adhesives with validated shear strength values that meet or exceed industry standards.
-
Biocompatibility: Given the nature of dental applications, adhesives must be biocompatible, meaning they should not elicit an adverse reaction from the body. Regulatory compliance with standards such as ISO 10993 is essential. Buyers must ensure that the adhesives they procure have been tested for biocompatibility to avoid complications post-surgery.
Common Trade Terminology
Understanding trade terminology is vital for navigating the procurement process effectively. Here are some key terms that B2B buyers should familiarize themselves with:
-
OEM (Original Equipment Manufacturer): This term refers to companies that produce parts or equipment that may be marketed by another manufacturer. In the context of dental implant adhesives, buyers may work directly with OEMs to ensure that the adhesives meet specific requirements and standards.
-
MOQ (Minimum Order Quantity): This is the smallest quantity of a product that a supplier is willing to sell. Understanding MOQ is essential for budgeting and inventory management, particularly for smaller dental practices or distributors that may not need large volumes.
-
RFQ (Request for Quotation): An RFQ is a document that a buyer sends to suppliers to request pricing and other details for specific products. Issuing an RFQ for dental implant adhesives allows buyers to compare prices, specifications, and terms across multiple suppliers, facilitating informed purchasing decisions.
-
Incoterms (International Commercial Terms): These are internationally recognized rules that define the responsibilities of sellers and buyers in international transactions. Familiarity with Incoterms is critical for understanding shipping costs, risk management, and delivery responsibilities, particularly when sourcing adhesives from different regions.
-
Lead Time: This refers to the time taken from placing an order until the product is received. In dental practices, where timely procedures are crucial, understanding lead time helps in planning and scheduling. Buyers should inquire about lead times when negotiating contracts.
-
Certification and Compliance: This encompasses the standards and regulations that the adhesives must meet, such as ISO certifications. Buyers should prioritize suppliers who can provide evidence of compliance to ensure that the products are safe and effective for dental use.
By grasping these technical properties and trade terms, B2B buyers can make informed decisions when sourcing dental implant adhesives, enhancing both operational efficiency and patient outcomes.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the dental implant adhesive Sector
Market Overview & Key Trends
The dental implant adhesive market is experiencing significant growth driven by several global factors. An increasing aging population and rising prevalence of dental disorders are compelling healthcare systems to adopt advanced dental solutions. In regions like Africa and South America, there is a surge in demand for affordable and effective dental products, while Europe and the Middle East are seeing a shift towards premium solutions that offer superior efficacy and reliability.
Emerging trends in sourcing technology, such as digital supply chain management and e-procurement platforms, are transforming how international buyers approach procurement. These technologies enhance transparency, streamline operations, and provide real-time data analytics, allowing buyers to make informed decisions. Additionally, the rise of direct-to-consumer models is reshaping traditional distribution channels, particularly in Europe, where buyers are increasingly looking for manufacturers who can deliver directly to clinics and hospitals.
Market dynamics are also influenced by regulatory changes aimed at improving product safety and efficacy. Buyers must stay abreast of these regulations, as compliance can significantly impact sourcing strategies. For instance, in the European Union, adherence to the Medical Device Regulation (MDR) is critical, while similar regulatory frameworks are emerging in other regions. Understanding these dynamics will empower B2B buyers to make strategic choices that align with their business goals and regional market demands.
Sustainability & Ethical Sourcing in B2B
As sustainability becomes a central concern for consumers and businesses alike, the dental implant adhesive sector is not exempt. The environmental impact of manufacturing processes and the materials used in dental adhesives is under scrutiny. B2B buyers are increasingly prioritizing suppliers who demonstrate a commitment to reducing their carbon footprint and utilizing sustainable materials.
The importance of ethical supply chains cannot be overstated. Buyers should seek out manufacturers that practice transparency in their sourcing processes, ensuring that materials are obtained responsibly and without harm to communities. Certifications such as ISO 14001 for environmental management and FSC (Forest Stewardship Council) for sustainable materials can serve as indicators of a supplier’s commitment to ethical practices.
Investing in ‘green’ certifications and sustainable materials not only aligns with global sustainability goals but also enhances brand reputation. As consumers become more environmentally conscious, businesses that prioritize sustainability in their sourcing decisions are more likely to gain a competitive edge in the marketplace. Buyers should actively inquire about a supplier’s sustainability initiatives and certifications to ensure they are making responsible purchasing decisions.
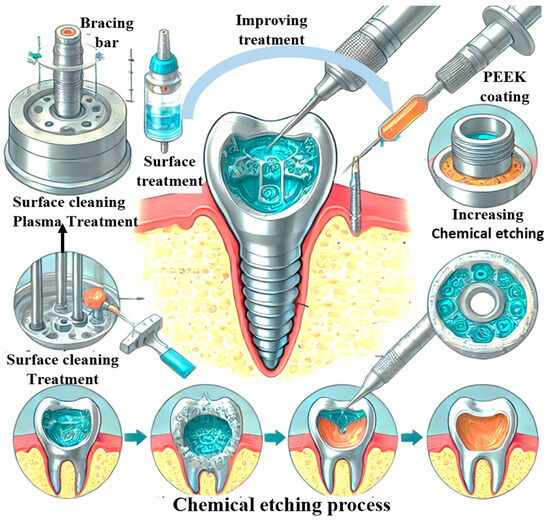
Illustrative Image (Source: Google Search)
Brief Evolution/History
The dental implant adhesive market has evolved significantly over the past few decades. Initially dominated by simple formulations, the industry has witnessed a shift towards more sophisticated adhesives that offer improved bonding strength and biocompatibility. Innovations in polymer chemistry and manufacturing processes have led to the development of adhesives that not only secure dental implants but also promote healing and integration with bone tissues.
In recent years, the introduction of digital technologies in the dental sector has further accelerated this evolution. The integration of 3D printing and digital impressions has opened new avenues for custom adhesive solutions tailored to individual patient needs. This historical trajectory highlights the importance of staying current with technological advancements and market shifts, enabling B2B buyers to make informed decisions that enhance their offerings in the competitive dental market.
Related Video: International Trade Explained
Frequently Asked Questions (FAQs) for B2B Buyers of dental implant adhesive
-
What should I consider when vetting suppliers of dental implant adhesive?
When vetting suppliers, prioritize their experience in the dental industry and their reputation among existing clients. Request references and case studies that demonstrate their reliability and product efficacy. Additionally, check for compliance with international standards, such as ISO certifications, to ensure quality and safety. Consider their manufacturing capabilities, as well as their ability to provide consistent supply and support. Engaging in direct communication can also help assess their responsiveness and willingness to collaborate on potential customization. -
Can dental implant adhesive be customized to meet specific needs?
Yes, many suppliers offer customization options for dental implant adhesive to cater to the unique requirements of different markets or applications. Engage with potential suppliers early in the negotiation process to discuss formulation adjustments, packaging preferences, or branding opportunities. Ensure that the supplier has a robust R&D department capable of supporting these customizations. Document any agreements in your contracts to avoid misunderstandings later in the partnership. -
What are typical minimum order quantities (MOQs) and lead times for dental implant adhesive?
MOQs can vary significantly between suppliers, often depending on their production scale and your specific requirements. Generally, MOQs for dental adhesive products range from 500 to 1,000 units. Lead times can also differ, typically ranging from 4 to 12 weeks depending on the supplier’s capacity and the complexity of your order. Always clarify these details upfront and consider negotiating MOQs that align with your business needs, especially if you are a new buyer or testing the market. -
What payment terms should I expect when sourcing dental implant adhesive internationally?
Payment terms can vary widely, but typical arrangements include upfront payments, letters of credit, or installment payments based on shipment milestones. For international transactions, it’s essential to establish clear terms that protect both parties. Be aware of currency exchange rates and transaction fees that may apply. Consider using escrow services for larger orders to mitigate risks. Always consult with financial experts to choose the most favorable terms that align with your cash flow and business strategy. -
What quality assurance measures should I verify from suppliers?
Quality assurance is critical in the dental industry. Ensure that your supplier has established quality control protocols, including testing for biocompatibility and adherence to regulatory standards. Request documentation of their quality assurance processes, such as batch testing reports and certifications from recognized authorities. Additionally, inquire about their recall procedures and how they handle product defects. This diligence will help protect your business from potential liabilities and ensure patient safety. -
What certifications should I look for in dental implant adhesive products?
When sourcing dental implant adhesive, look for certifications that demonstrate compliance with international health and safety standards. Key certifications include ISO 13485 for medical devices, CE marking for the European market, and FDA approval in the U.S. These certifications indicate that the product has been rigorously tested and meets quality benchmarks. Always request copies of these certifications from suppliers and verify their authenticity to ensure compliance with local regulations in your target market. -
How can I manage logistics and shipping for international orders of dental implant adhesive?
Effective logistics management is crucial for timely delivery of dental implant adhesive. Work closely with your supplier to understand their shipping methods, lead times, and any potential customs requirements. Utilize freight forwarders experienced in medical products to navigate the complexities of international shipping. Establish clear communication channels for tracking shipments and addressing any potential delays. Additionally, consider using insurance for high-value shipments to mitigate risks associated with loss or damage during transit. -
What steps can I take to resolve disputes with suppliers?
Disputes can arise over various issues, including product quality, delivery delays, or contractual obligations. To effectively manage these disputes, maintain open lines of communication with your supplier and attempt to resolve issues amicably first. Include a dispute resolution clause in your contracts, outlining processes for mediation or arbitration to avoid lengthy legal battles. Document all communications and agreements meticulously, as this can provide evidence should formal resolution methods be necessary. Building strong relationships with suppliers can also help preemptively address potential conflicts.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for dental implant adhesive
The strategic sourcing of dental implant adhesives is pivotal for international B2B buyers aiming to enhance their product offerings and operational efficiency. Key takeaways emphasize the importance of understanding regional market dynamics, supplier reliability, and compliance with local regulations. Engaging with suppliers who prioritize innovation and sustainability can lead to competitive advantages, particularly in emerging markets like Africa and South America, where demand for high-quality dental products is on the rise.
Value of Strategic Sourcing:
– Cost Efficiency: Leveraging strategic partnerships can lead to better pricing structures and reduced operational costs.
– Quality Assurance: Sourcing from reputable suppliers ensures adherence to quality standards, which is critical for maintaining customer trust.
– Supply Chain Resilience: Diversifying suppliers mitigates risks associated with geopolitical tensions and market fluctuations.
Looking ahead, B2B buyers must remain proactive in their sourcing strategies to adapt to evolving market needs and technological advancements. By prioritizing strategic sourcing, businesses can not only meet current demands but also position themselves for future growth in the dental implant adhesive market. Take the next step: evaluate your sourcing strategies today to unlock new opportunities in this dynamic sector.
