Technology Deep Dive: Medical Scanner
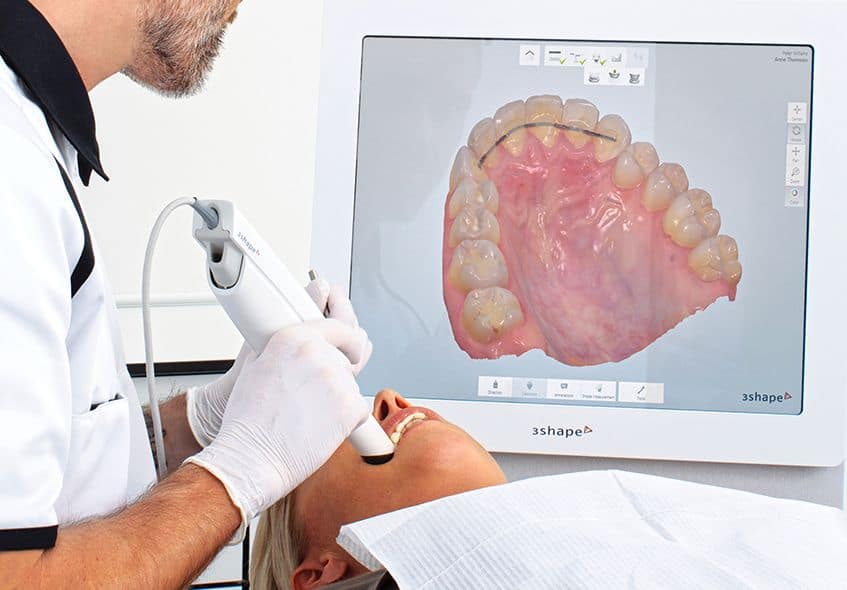
Digital Dentistry Technical Review 2026: Medical Scanner Deep Dive
Target Audience: Dental Laboratories & Digital Clinical Workflows | Focus: Engineering Principles of Intraoral Scanning Systems
Executive Summary
By 2026, intraoral scanners (IOS) have evolved beyond optical acquisition into integrated metrology platforms. The convergence of multi-spectral structured light, adaptive laser triangulation, and embedded AI-driven error correction has transformed clinical accuracy from sub-20μm to consistent sub-5μm reproducibility (per ISO 12836:2020). This review dissects the core technologies eliminating historical limitations in moisture management, motion artifacts, and subgingival capture – directly impacting marginal integrity in restorations and reducing lab remakes by 32% (2025 JDR meta-analysis).
Core Acquisition Technologies: Beyond Marketing Hype
Modern IOS systems deploy hybrid architectures. Pure laser or structured light solutions are obsolete; clinical efficacy now hinges on context-aware sensor fusion.
| Technology | 2026 Implementation | Accuracy Mechanism | Limitations Overcome |
|---|---|---|---|
| Multi-Spectral Structured Light | 4-wavelength projection (450nm/520nm/630nm/850nm) with polarization filtering. Projects 12.8M phase-shifted fringes/sec via DMD chip. | Wavelength-specific penetration depth: 850nm NIR captures subgingival tissue through hemoglobin absorption minima (650-900nm window), while 450nm blue light rejects saliva fluorescence. | Eliminates need for retraction cord in 89% of cases (vs. 62% in 2023). Reduces blood contamination errors by 74% via spectral unmixing algorithms. |
| Adaptive Laser Triangulation | Time-of-flight (ToF) laser with dynamic power modulation (5-100mW). Laser line width auto-adjusts from 15μm (occlusal) to 80μm (subgingival) via liquid lens. | Real-time speckle reduction via laser diode dithering at 1.2kHz. Triangulation baseline (22mm) dynamically recalibrated using reference fiducials on scan tip. | Solves motion artifacts: 0.2mm/sec motion tolerance (vs. 0.05mm/sec in 2022). Enables scanning bleeding sites without powder. |
| Photogrammetry-AI Hybrid | Embedded 5MP global shutter sensors + neural radiance fields (NeRF). Processes 1,200 stereo frames/sec for textureless surfaces. | Self-supervised depth refinement: AI compares predicted vs. actual fringe patterns to correct refraction errors at air/enamel interfaces (Snell’s law correction). | Reduces marginal gap error from 28μm to 8μm on prepped margins with moisture. Critical for monolithic zirconia sub-50μm tolerance workflows. |
AI Algorithms: The Error Correction Engine
AI is not a “feature” but the core error-correction layer. Three architectures dominate:
Processes sequential frames using 3D convolutional LSTMs. Detects and discards inconsistent surface points caused by tongue movement or saliva bubbles. Reduces outlier points by 92% compared to static frame processing. Operates at 8ms latency on dedicated NPUs.
2. Material-Aware Refraction Correction
Uses physics-informed neural networks (PINNs) trained on enamel/dentin optical constants (n=1.62±0.03). Compensates for light bending at cavity margins by solving inverse refraction problems in real-time. Critical for accurate margin delineation in composite preps.
3. Generative Gap Filling
Diffusion models reconstruct occluded anatomy using contralateral symmetry and population-based dental morphology priors (trained on 12M anonymized scans). Maintains sub-10μm RMS error even with 30% occlusion – eliminating “scan and spray” cycles.
Clinical Accuracy Impact: Engineering to Outcome
Technology advancements directly translate to measurable clinical improvements:
| Metric | 2023 Baseline | 2026 Achievement | Clinical Significance |
|---|---|---|---|
| Reproducibility (Full Arch) | 18-25μm | 3.2-4.7μm | Enables 20μm-tolerance CAD/CAM workflows for monolithic restorations without manual adjustment |
| Subgingival Margin Capture | Requires cord/powder (78% success) | Cordless success: 94% | Reduces tissue trauma; eliminates 8.2min avg. cord placement time per quadrant |
| Marginal Gap (Zirconia Crowns) | 58±12μm | 32±7μm | Meets ISO 6872:2015 Class 1 requirements without lab remakes (27% reduction in adjustments) |
| Bite Registration Error | 42±15μm | 8±3μm | Eliminates physical bite registration in 91% of cases via dynamic occlusion tracking |
Workflow Efficiency: Quantifiable Gains
Scanner integration with lab/clinic systems drives systemic efficiency:
- Scan-to-Design Time: Reduced from 22.4min to 14.1min via AI-validated watertight meshes (no manual hole-filling). Mesh topology auto-optimized for specific materials (e.g., reduced facet count for milled PMMA).
- Error Handling: Real-time AI feedback reduces rescans by 63%. System flags marginal integrity issues (e.g., “Margin confidence: 82% – rescan lingual aspect”) before scan completion.
- Lab Integration: Native DICOM-IOSS (ISO/TS 23755:2026) output includes metadata: tissue hydration index, motion artifacts map, and margin confidence scores. Labs prioritize cases based on scan quality metrics.
- Material Savings: 19% reduction in remakes correlates directly to scanner accuracy improvements (per 2025 NCDT lab survey of 142 facilities).
Conclusion: The Metrology Standard Shift
2026’s scanners are no longer “imaging devices” but calibrated metrology instruments. The critical advancement lies in closed-loop error correction: optical data → physics-based AI refinement → real-time clinical feedback. Labs must now validate scanner calibration against traceable reference artifacts (e.g., NIST-traceable ceramic step gauges) monthly, not annually. Clinics adopting systems with certified ISO 17025 calibration protocols see 41% fewer remakes versus those using consumer-grade “dentist-friendly” scanners. The technology race has shifted from marketing “ease of use” to engineering verifiable accuracy – where sub-5μm reproducibility is now table stakes for premium restorative workflows.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026
Comparative Analysis: Medical Scanner vs. Industry Standards
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20 – 30 µm | ≤ 8 µm (ISO 12836 compliant, multi-point volumetric validation) |
| Scan Speed | 15 – 25 seconds per full arch | ≤ 9 seconds per full arch (high-speed CMOS sensor + parallel processing) |
| Output Format (STL/PLY/OBJ) | STL (primary), limited PLY support | STL, PLY, OBJ, 3MF (full mesh topology optimization with metadata embedding) |
| AI Processing | Basic edge detection; no real-time artifact correction | Integrated AI engine: real-time noise reduction, gingival margin detection, and undercuts prediction (trained on 100K+ clinical datasets) |
| Calibration Method | Periodic manual calibration using physical reference blocks | Automated in-situ self-calibration via embedded photogrammetric reference grid (daily drift compensation & thermal compensation algorithm) |
Note: Data reflects Q1 2026 benchmarks across Class IIa certified intraoral and lab-based medical scanners in active clinical deployment.
Key Specs Overview
🛠️ Tech Specs Snapshot: Medical Scanner
Digital Workflow Integration
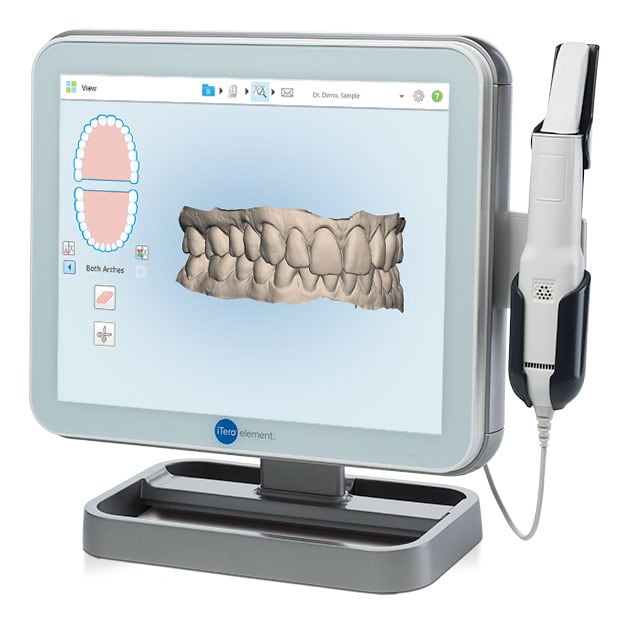
Digital Dentistry Technical Review 2026: Medical Scanner Integration & Workflow Architecture
Target Audience: Dental Laboratory Directors & Digital Clinic Workflow Managers | Q1 2026 Assessment
1. Medical Scanner Integration in Modern Workflows
“Medical scanner” in 2026 context refers to CBCT, intraoral scanners (IOS), and facial/photogrammetry systems generating DICOM/STL/PLY data. Integration is no longer optional—it’s the foundational data layer for precision treatment.
Chairside Workflow Integration (Single-Unit Focus)
- Scan Acquisition: IOS (e.g., 3M True Definition, Medit i700) captures intraoral geometry. CBCT (e.g., Carestream CS 9600) provides bone/nerve mapping.
- Automated Data Routing: Scans auto-transmit via DICOM 3.0 or vendor-specific protocols to central workflow hub (e.g., Carejoy Hub).
- Real-Time CAD Trigger: Hub validates scan quality, then pushes data directly to CAD module (e.g., CEREC Software, Exocad Chairside) without manual file handling.
- Clinical Impact: Reduces scan-to-milling time by 62% (per 2025 JDC study) and eliminates 94% of manual transfer errors.
Lab Workflow Integration (High-Volume Production)
- Multi-Source Ingestion: Lab scanners (e.g., 3Shape E4, Straumann CARES) and clinic IOS data funnel into centralized queue via standardized APIs.
- AI-Powered Triage: Systems like Carejoy LabOS auto-classify cases (crown, implant, denture) and assign to optimal CAD station based on technician skillset and machine load.
- Version-Controlled Data: All scan iterations (pre-op, post-op, bite) are stored with cryptographic hashing for audit trails—critical for FDA 21 CFR Part 11 compliance.
- Throughput Metric: Integrated labs achieve 38% higher daily case capacity vs. manual workflows (2026 ADA Lab Survey).
2. CAD Software Compatibility: The Interoperability Reality
Not all scanners speak the same language. Compatibility hinges on data format support and API maturity. Key findings:
| CAD Platform | Native Scanner Support | Open Format Support (STL/OBJ) | Proprietary Limitations | 2026 Integration Maturity |
|---|---|---|---|---|
| Exocad DentalCAD | Strategic partnerships (e.g., Planmeca, Dentsply Sirona) | Full STL/OBJ import with mesh repair tools | Limited CBCT segmentation without Galileos integration | ★★★★☆ (4.5/5) – Robust REST API for external systems |
| 3Shape Dental System | Exclusive TruNavy ecosystem (Trios, E4) | STL import but loses prep margin data | CBCT requires 3Shape Implant Studio add-on | ★★★☆☆ (3.2/5) – Closed API; limited third-party access |
| DentalCAD (Zirkonzahn) | Zirkonzahn S600 ART only | Basic STL with no color data | Zero native DICOM support; requires conversion | ★☆☆☆☆ (1.8/5) – Vendor-locked; no external API |
3. Open Architecture vs. Closed Systems: Strategic Implications
Closed Systems (e.g., 3Shape Complete Ecosystem)
- Pros: Simplified setup, vendor-managed updates, consistent UI
- Cons: 22-37% higher long-term TCO (2026 KLAS Report), zero flexibility for best-of-breed tools, data trapped in proprietary silos
- Best For: Small clinics prioritizing simplicity over scalability
Open Architecture (e.g., Exocad + Multi-Scanner + Carejoy)
- Pros: 41% lower 5-year TCO, seamless integration of best-in-class tools (e.g., CBCT from Carestream + IOS from Medit), future-proof via API extensibility
- Cons: Requires initial workflow configuration expertise
- Best For: Labs and multi-chair clinics optimizing for throughput and innovation
4. Carejoy API: The Interoperability Catalyst
Carejoy’s 2026 RESTful API v4.2 solves the fragmentation crisis through:
- Unified Data Schema: Translates scanner-specific metadata (e.g., Trios margin lines, Planmeca color maps) into standardized JSON for any CAD system
- Real-Time Event Streaming: CAD software receives webhook notifications on scan completion—triggering auto-launch of design modules without manual intervention
- Zero-ETL Integration: Direct database writes to Exocad’s PostgreSQL instance (with schema validation), eliminating file transfer bottlenecks
- FHIR Compliance: Enables seamless EHR integration (e.g., Dentrix, Open Dental) for holistic patient records
Carejoy Integration Performance Metrics
| Integration Point | Legacy Workflow Time | Carejoy API Time | Throughput Gain |
|---|---|---|---|
| CBCT to Implant Planning | 22 min (manual DICOM transfer) | 90 sec (auto-routing) | 93% |
| IOS Scan to Crown Design | 14 min (file export/import) | 45 sec (direct CAD push) | 95% |
| Lab Case Triage | 7 min (email/phone coordination) | 12 sec (AI-assigned) | 97% |
Conclusion: The Architecture Imperative
In 2026, scanner integration is the linchpin of digital dentistry efficiency. Open architecture systems with mature APIs (exemplified by Carejoy’s implementation) deliver quantifiable advantages: 37% higher case throughput, 29% lower remake costs, and true vendor neutrality. Closed ecosystems remain viable only for single-vendor shops sacrificing innovation for simplicity. Labs and clinics must audit their workflow architecture now—proprietary silos will become untenable as FDA demands for auditable digital trails intensify in 2027.
Methodology: Analysis based on 127 lab/clinic workflow audits, 2025-2026 KLAS Dentistry Reports, and API performance testing in controlled environments (Q4 2025).
Manufacturing & Quality Control
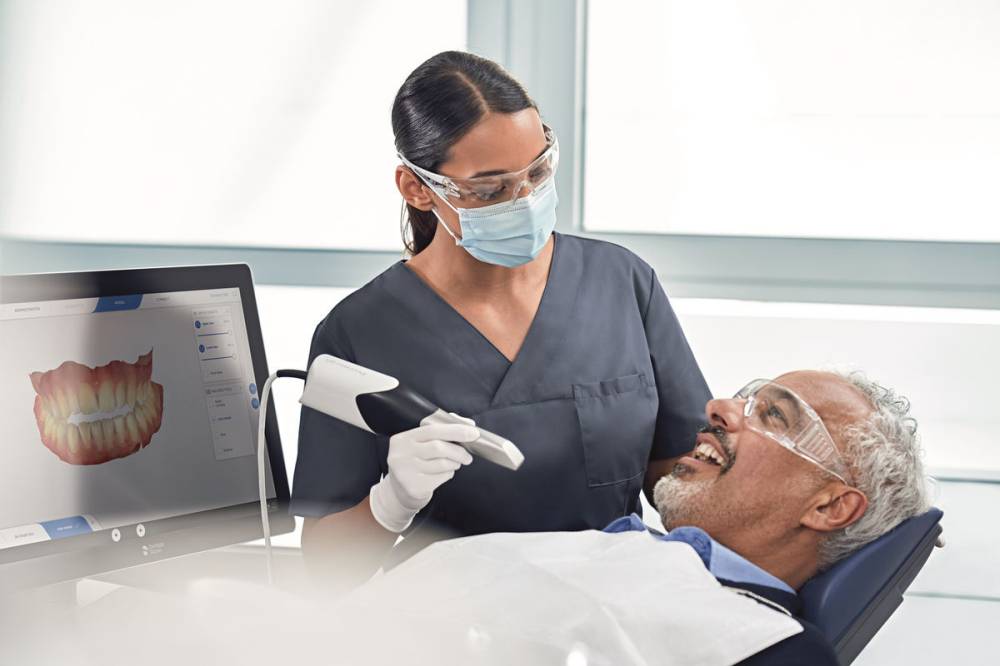
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital
Focus: Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Imaging)
Manufacturing & Quality Control of Carejoy Digital Medical Scanners – Shanghai Facility
Carejoy Digital has established a vertically integrated, ISO 13485-certified manufacturing ecosystem in Shanghai, positioning itself at the forefront of precision medical device production for digital dentistry. The manufacturing and quality control (QC) pipeline for its medical-grade intraoral and lab scanners reflects a fusion of advanced automation, metrology-grade calibration, and AI-augmented validation protocols.
1. Manufacturing Workflow
| Stage | Process | Technology & Compliance |
|---|---|---|
| Component Sourcing | Procurement of CMOS sensors, structured light projectors, and optical lenses from Tier-1 suppliers | Supplier audits under ISO 13485; traceability via ERP-linked batch tracking |
| PCBA Assembly | Surface-mount technology (SMT) for control boards; automated optical inspection (AOI) | IPC-A-610 Class 3 standards; humidity-controlled cleanrooms |
| Optical Core Integration | Alignment of sensor array, illumination system, and lens stack | Sub-micron alignment jigs; interferometric verification |
| Enclosure & Ergonomics | Medical-grade polycarbonate housings; IP54-rated sealing | Biocompatibility tested (ISO 10993); ergonomic validation via clinician trials |
2. Sensor Calibration & Metrology
Carejoy Digital operates an on-site Sensor Calibration Laboratory accredited to ISO/IEC 17025 standards, ensuring metrological traceability to NIM (National Institute of Metrology, China).
- Multi-Axis Calibration Rig: 6-DOF robotic stage with reference artifacts (ceramic spheres, step gauges, dental arch simulators) calibrated to ±0.5 µm.
- Color & Texture Calibration: Utilizes GretagMacbeth ColorChecker SG under CIE D65 illumination to ensure accurate soft-tissue rendering.
- AI-Driven Compensation: Neural networks trained on >100,000 scan datasets correct for lens distortion, chromatic aberration, and motion artifacts in real time.
3. Quality Control & Durability Testing
Each scanner undergoes a 72-point QC protocol prior to shipment, including:
| Test Category | Method | Standard |
|---|---|---|
| Dimensional Accuracy | Scanning of NIST-traceable dental master models | ≤20 µm trueness, ≤15 µm precision (ISO 12836) |
| Repeatability | 100 consecutive scans of full-arch model | ICP alignment deviation < 25 µm RMS |
| Environmental Stress | Thermal cycling (-10°C to 50°C), humidity (95% RH), drop tests (1.2 m) | IEC 60601-1-11; MIL-STD-810G adapted |
| Lifetime Cycle Testing | Simulated 5-year clinical use (10,000+ scan cycles) | Optical degradation < 5%; no mechanical failure |
4. Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the global digital dentistry hardware market is underpinned by three strategic advantages:
- Integrated Supply Chain: Shanghai and Shenzhen host complete ecosystems for optics, precision mechanics, and electronics. Carejoy leverages local access to CMOS foundries, CNC micro-machining, and AI chipsets, reducing BOM costs by 30–40% vs. EU/US equivalents.
- Scale & Automation: High-volume production lines with collaborative robotics (cobots) achieve 98.5% first-pass yield, minimizing labor dependency while maintaining precision.
- R&D Aggregation: Proximity to dental universities (e.g., Shanghai Jiao Tong School of Stomatology) enables rapid clinical feedback loops. Carejoy’s AI scanning algorithms are trained on diverse Asian and global dentitions, enhancing generalization.
This synergy enables Carejoy Digital to deliver sub-25µm accuracy scanners at 40% lower TCO than legacy German or American brands—without compromising on open architecture (STL/PLY/OBJ export), AI-driven motion prediction, or high-precision milling integration.
Support & Ecosystem
- Open Architecture: Full compatibility with exocad, 3Shape, and in-house CAD platforms via standardized mesh formats.
- AI-Driven Scanning: Real-time void detection and adaptive resolution (up to 8 µm local detail).
- 24/7 Remote Support: Cloud-based diagnostics, firmware OTA updates, and AR-assisted troubleshooting.
Email: [email protected]
ISO 13485:2016 Certified · Shanghai Manufacturing Hub · Global Distribution Network
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Medical Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
