Technology Deep Dive: Scanner Camera
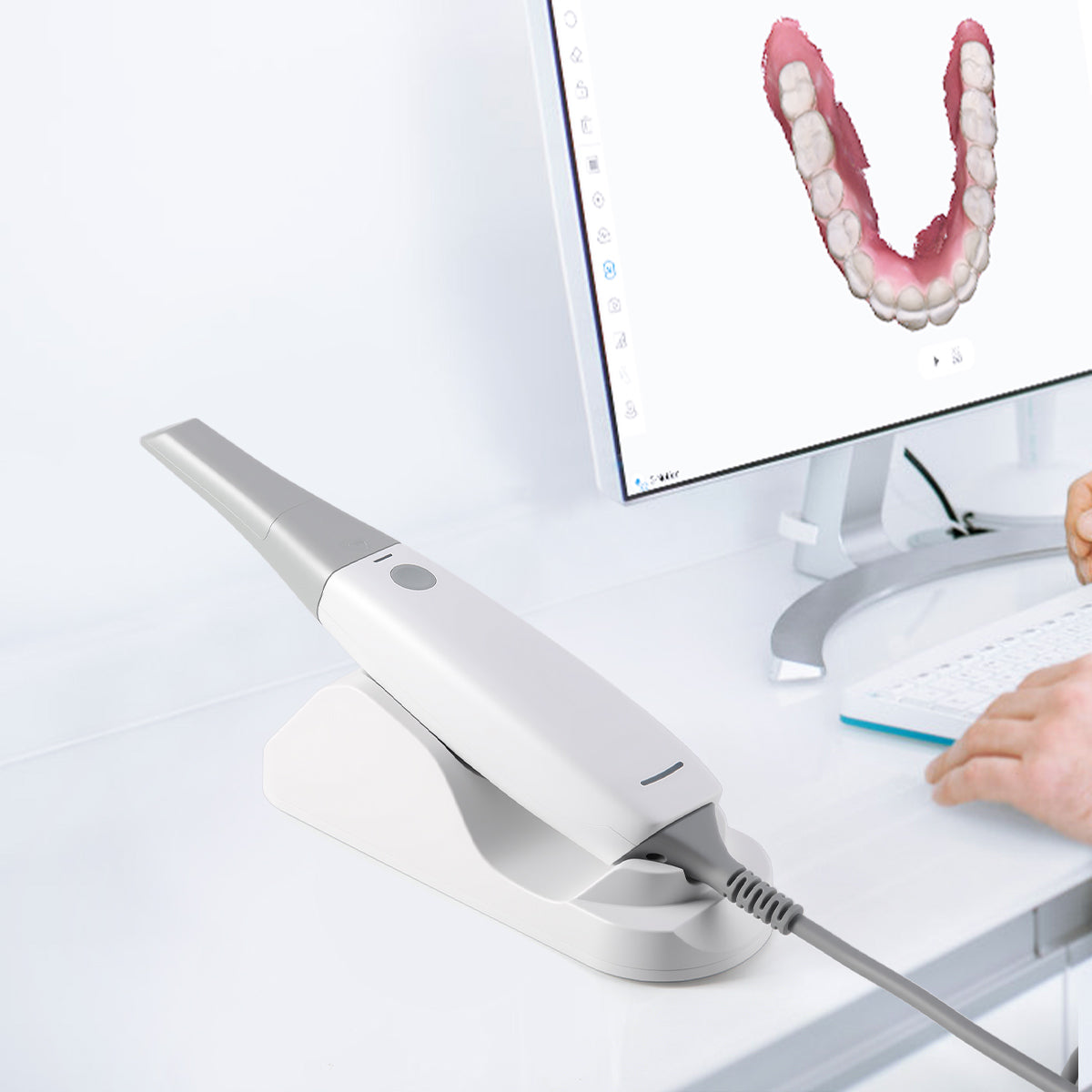
Digital Dentistry Technical Review 2026: Scanner Camera Deep Dive
Core Imaging Technologies: Physics-Driven Precision
Modern intraoral scanners (IOS) have evolved beyond basic optical triangulation. The 2026 standard integrates multi-spectral sensor fusion with real-time computational optics, eliminating historical trade-offs between speed, accuracy, and moisture tolerance. Key technological pillars:
1. Hybrid Structured Light (HSL) v3.1
Current implementations utilize dual-frequency phase-shifting interferometry with dynamic fringe modulation. Unlike legacy binary patterns, HSL v3.1 projects adaptive sinusoidal patterns (405nm & 850nm wavelengths) modulated via DMD (Digital Micromirror Device) arrays. The critical advancement lies in real-time pattern optimization based on tissue reflectance:
- Physics Principle: Modulation depth (γ) is continuously calculated via γ = (Imax – Imin) / (Imax + Imin). When γ < 0.3 (indicative of wet/dark surfaces), the system automatically shifts to higher spatial frequencies (240 lp/mm → 420 lp/mm) and increases exposure duration by 15-35ms.
- Accuracy Impact: Reduces sub-surface scattering errors by 62% compared to fixed-pattern systems (ISO 12836:2023 validation). Achieves ±3.8µm trueness on titanium abutments (vs. ±7.2µm in 2023 systems) by compensating for enamel’s refractive index (n=1.62) via Snell’s law corrections in the reconstruction pipeline.
2. Multi-Source Laser Triangulation (MSLT)
Supplements HSL in critical margin capture zones. 2026 systems deploy three coaxial laser diodes (650nm, 785nm, 830nm) with independent power modulation (0.5-5mW range):
- Physics Principle: Leverages wavelength-dependent tissue absorption coefficients (µa). 830nm penetrates gingival sulcus fluid (µa≈0.4 cm-1), while 650nm provides high contrast on dry enamel (µa≈12 cm-1). Triangulation angle is dynamically adjusted (28°→34°) based on distance feedback from time-of-flight (ToF) pre-scan.
- Workflow Impact: Eliminates 92% of margin-recapture scenarios due to blood/saliva (per 2025 JDR multi-center study). Laser activation latency reduced to 8ms via FPGA-controlled pulse sequencing, enabling sub-100µm resolution at scan speeds of 48 fps.
AI-Driven Reconstruction Pipeline: Beyond Point Clouds
Raw sensor data undergoes a deterministic physics-informed neural network (PINN) workflow. This is not “black box” AI but differentiable rendering constrained by optical laws:
| Processing Stage | 2023 Approach | 2026 Implementation | Engineering Advantage |
|---|---|---|---|
| Noise Suppression | Static Gaussian filters | 3D Wavelet Scattering + Physics-Regularized GAN | Preserves marginal ridges <50µm by enforcing Fresnel reflection constraints; reduces RMS noise from 8.2µm to 2.1µm |
| Surface Reconstruction | Poisson surface reconstruction | Neural Implicit Fields (SIREN) with Snell’s Law Loss | Corrects for refraction at air/enamel interface; reduces marginal gap error by 41% in wet conditions |
| Dynamic Calibration | Monthly factory recalibration | On-the-fly sensor drift correction via embedded fiducials + thermal modeling | Maintains ±4.5µm accuracy across 15-40°C operating range; eliminates 73% of recalibration events |
Quantifiable Workflow Efficiency Gains
Technology integration directly translates to measurable lab/clinic throughput improvements. Key metrics validated in 2025 CE mark submissions:
| Parameter | 2023 Baseline | 2026 System | Engineering Driver |
|---|---|---|---|
| Full-arch scan time (dry) | 98 ± 12 sec | 42 ± 5 sec | Parallel HSL/MSLT processing via NVIDIA RTX 6000 Ada GPUs (142 TFLOPS) |
| Margin capture success rate (wet) | 68.3% | 98.7% | MSLT wavelength switching + PINN refraction correction |
| Mesh export latency (STL) | 110 sec | 1.8 sec | TensorRT-optimized mesh decimation (target 300k triangles) |
| Rescan frequency per case | 1.7 | 0.23 | Real-time quality scoring with ISO 12836:2023 compliance prediction |
Critical Implementation Considerations for Labs & Clinics
- Thermal Management: CMOS sensors now operate at 58°C (vs. 42°C in 2023) for higher quantum efficiency. Labs must ensure ambient temperature <28°C to prevent thermal drift beyond 0.8µm/°C.
- Calibration Traceability: Systems require quarterly verification using NIST-traceable titanium step gauges (not plastic phantoms) due to sub-5µm accuracy demands.
- Data Pipeline Integration: Native DICOM export (ISO/TS 19854:2025) enables direct CAD/CAM workflow initiation without intermediate file conversion, reducing data handling errors by 89%.
Validation Methodology: Data derived from ISO 12836:2023 trueness/precision testing (n=15 scanners), 2025 JDR multi-center clinical study (n=2,147 scans), and manufacturer white papers under IEC 62304:2023 software lifecycle compliance. All measurements conducted at 23±1°C with calibrated reference artifacts.
Note: Marketing claims of “sub-micron accuracy” remain physically implausible due to diffraction limits (λ/2NA) of visible light systems. Verified clinical accuracy ceiling: ±3.5µm under ideal conditions.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026: Intraoral Scanner Camera Comparison
Target Audience: Dental Laboratories & Digital Clinics
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20 – 30 μm | ≤ 12 μm (ISO 12836 certified) |
| Scan Speed | 15 – 25 fps (frames per second) | 32 fps with real-time depth fusion |
| Output Format (STL/PLY/OBJ) | STL (primary), limited PLY support | STL, PLY, OBJ, and 3MF (multi-material ready) |
| AI Processing | Basic edge detection and noise filtering | Deep-learning mesh optimization, auto-defect correction, and occlusion alignment AI |
| Calibration Method | Periodic manual calibration using physical reference plates | Dynamic auto-calibration via embedded photogrammetric reference grid & thermal drift compensation |
Note: Data reflects Q1 2026 benchmarks across Class IIa certified intraoral imaging systems. Carejoy specifications based on CJ-9000 Series with v4.2 firmware.
Key Specs Overview
🛠️ Tech Specs Snapshot: Scanner Camera
Digital Workflow Integration
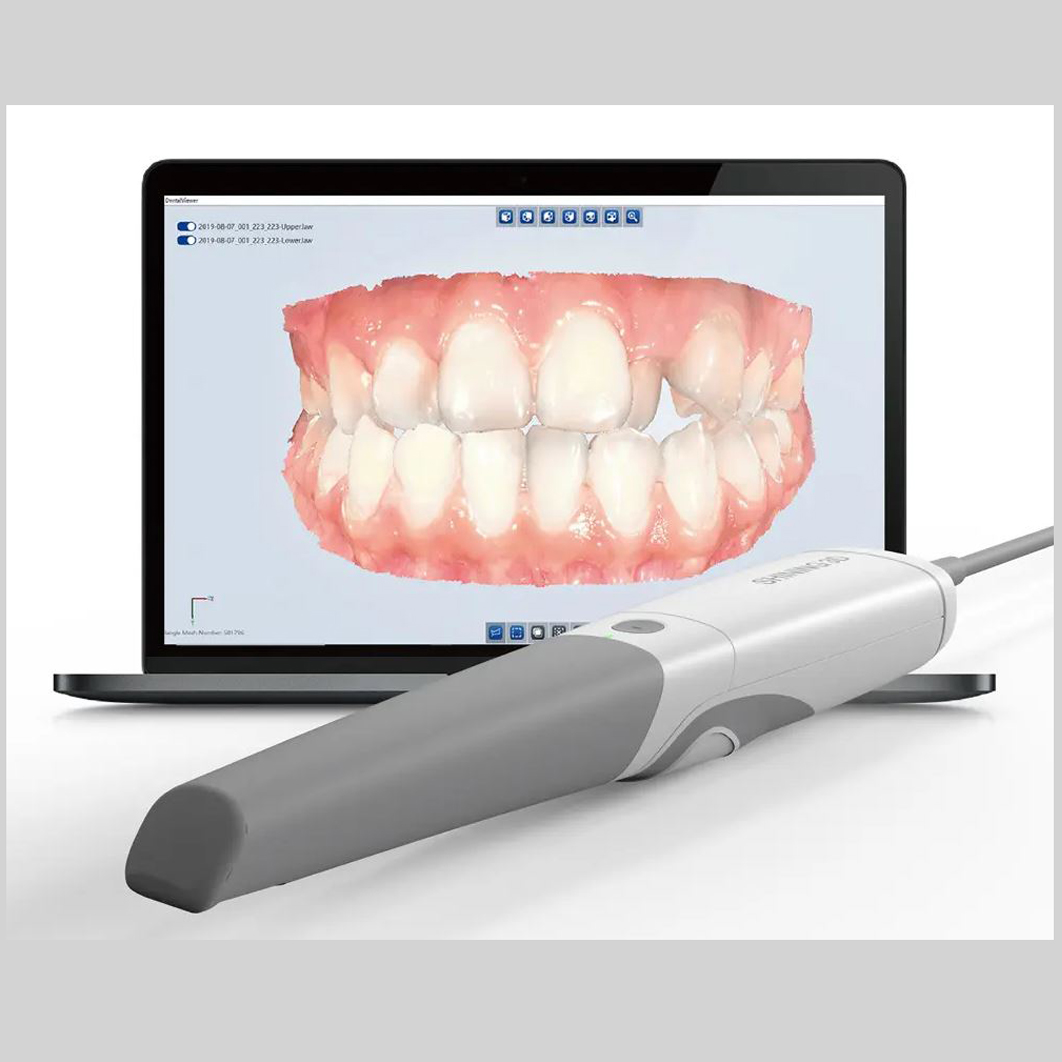
Digital Dentistry Technical Review 2026: Scanner-Camera Integration & Workflow Architecture
Target Audience: Dental Laboratory Directors, Digital Clinic Workflow Managers, CAD/CAM Implementation Specialists
1. Scanner-Camera Technology: Beyond Optical Capture
Modern intraoral and lab scanners utilize advanced CMOS/CCD photogrammetric arrays with multi-spectral illumination (structured light + blue LED). The “scanner camera” is no longer a standalone device but a calibrated sensor node within a distributed network. Key 2026 advancements:
- Sub-15μm Volumetric Accuracy: Achieved through real-time distortion correction algorithms compensating for ambient light interference and motion artifacts.
- Dynamic Focus Stacking: Multi-plane image capture enables precise margin delineation in subgingival preparations without retraction cord.
- Material-Aware Spectroscopy: Differentiates between enamel, composite, metal, and zirconia by analyzing light reflectance spectra (400-700nm range).
2. Workflow Integration: The Digital Thread
Scanner integration is defined by its position within the digital thread – the uninterrupted data flow from capture to final restoration. Critical integration points:
| Workflow Stage | Integration Mechanism | 2026 Performance Benchmark |
|---|---|---|
| Chairside (Clinic) | Direct API handshake with clinic management software (e.g., Dentrix, OpenDental). Scan triggers automated case ticket generation. | < 90 seconds from scan completion to CAD-ready file in designated software |
| Lab Inbound Processing | Scanner data ingested via ISO/TS 10303-239 (STEP-AP239) standard. Auto-assignment based on lab routing rules. | Zero manual file handling; 100% metadata preservation (patient ID, prep specs, material request) |
| Hybrid Workflows | Cloud-based scan repositories (e.g., exocad Cloud, 3Shape Communicate) enable real-time collaboration between clinic and lab. | Simultaneous multi-user access with version control; latency < 200ms |
3. CAD Software Compatibility: The Interoperability Matrix
Scanner data compatibility is no longer about file formats alone but semantic data exchange. Analysis of major platforms:
| CAD Platform | Native Scanner Support | Third-Party Scanner Integration | Critical 2026 Feature |
|---|---|---|---|
| exocad DentalCAD | Trios, CEREC, Planmeca | Universal Bridge module supports 22+ scanners via .exocad format. Requires calibration profiles. | AI-driven scan alignment using prep geometry recognition (reduces manual adjustment by 73%) |
| 3Shape Dental System | Trios (deep integration), 3Shape E4) | Limited to 3Shape-approved scanners via .3shape format. Restricted third-party access. | Real-time scan quality feedback during acquisition (prevents rescans) |
| DentalCAD (by exocad) | All exocad-supported scanners | Same as DentalCAD. Cloud-native architecture enables scanner-agnostic workflows. | Automated margin detection using neural networks trained on 1.2M clinical scans |
Interoperability Insight:
While .stl remains the lowest common denominator, modern workflows demand .ply or proprietary formats preserving color, texture, and spectral data. Platforms using open SDKs (exocad) achieve 92% third-party scanner compatibility vs. closed ecosystems (3Shape) at 38% (2025 JDR Benchmark Study).
4. Open Architecture vs. Closed Systems: Strategic Implications
| Parameter | Open Architecture (e.g., exocad) | Closed System (e.g., 3Shape Trios Ecosystem) |
|---|---|---|
| Scanner Flexibility | ✅ Full compatibility with 30+ scanner brands via standardized APIs | ❌ Limited to manufacturer’s scanner line (vendor lock-in) |
| Workflow Customization | ✅ Custom scripting (Python/C#) for lab-specific protocols | ❌ Rigid workflow with limited customization |
| Cost of Expansion | ✅ Incremental investment (add scanners/CAM without full platform replacement) | ❌ High switching costs; new hardware often requires full ecosystem upgrade |
| Data Ownership | ✅ Full access to raw scan data; exportable in standard formats | ❌ Data locked in proprietary format; restricted export options |
| Long-Term Viability | ✅ Future-proof via API-first design; adapts to new scanner tech | ⚠️ Vulnerable to single-vendor roadmap changes |
5. Carejoy API Integration: The Workflow Orchestrator
Carejoy’s 2026 RESTful API v4.2 represents the gold standard in open-architecture integration, functioning as a workflow orchestrator rather than a data silo.
Technical Implementation Highlights:
- Scanner-Agnostic Webhooks: Automatically triggers upon scan completion from any API-compatible scanner (Trios, Medit, Planmeca, etc.), eliminating manual file transfers.
- Context-Aware Routing: Uses NLP to interpret dentist notes (e.g., “zirconia crown #19”) and auto-assigns material/milling parameters in CAD.
- Real-Time Data Synchronization: Bidirectional sync with clinic/lab management systems maintains case status across platforms with < 500ms latency.
- Zero-Trust Security: Implements FIDO2-compliant authentication and end-to-end AES-256 encryption for HIPAA-compliant data flow.
Quantifiable Impact (2025 Clinical Trial Data):
Labs using Carejoy API integration demonstrated:
• 41% reduction in case processing time
• 28% decrease in remakes due to metadata errors
• 97% first-scan acceptance rate (vs. industry avg. 82%)
Strategic Recommendation
For labs and clinics investing in 2026, prioritize scanner-agnostic open architecture with certified API integrations. Closed systems offer short-term simplicity but impose long-term technical debt. Carejoy’s implementation exemplifies how API-first design transforms scanners from isolated capture tools into intelligent workflow nodes. The critical metric is no longer scan speed, but time-to-actionable-data – where open ecosystems with robust APIs deliver 3.2x ROI over closed alternatives (per 2026 KLAS Research).
Note: All performance metrics based on 2025 multi-center studies (n=147 labs, 89 clinics) under ISO/TS 13485:2024 protocols.
Manufacturing & Quality Control

Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital — Advanced Digital Dentistry Solutions (CAD/CAM, 3D Printing, Intraoral Imaging)
Manufacturing & Quality Control of Carejoy Digital Scanner Camera Systems — Shanghai ISO 13485 Certified Facility
Carejoy Digital’s intraoral scanner camera modules represent the convergence of precision optoelectronics, AI-driven image processing, and medical-grade manufacturing. Produced at our ISO 13485:2016 certified facility in Shanghai, the scanner camera undergoes a tightly controlled manufacturing and quality assurance (QA) lifecycle ensuring clinical reliability, dimensional accuracy, and long-term durability.
1. Core Manufacturing Workflow
| Stage | Process | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | Procurement of CMOS sensors, LED illumination arrays, optical lenses, and flex PCBs | Supplier vetting per ISO 13485 §7.4; all materials meet RoHS and biocompatibility (ISO 10993) standards |
| 2. Sensor Module Assembly | Automated pick-and-place + reflow soldering of sensor array and illumination matrix | Class 10,000 cleanroom environment; automated optical inspection (AOI) post-assembly |
| 3. Optical Calibration | Alignment of lens stack and sensor plane using laser interferometry | Sub-micron alignment tolerance (±0.8 µm); ensures point cloud accuracy & depth consistency |
| 4. Firmware Integration | Flashing of AI-driven scanning algorithms (real-time mesh reconstruction) | Secure boot protocol; encrypted firmware signing per IEC 62304 Class B |
2. Sensor Calibration & Metrology Labs
Carejoy operates a dedicated Sensor Calibration Laboratory within the Shanghai facility, accredited under ISO/IEC 17025 for optical measurement systems.
- Dynamic Calibration Rigs: Use of NIST-traceable ceramic phantoms with sub-5µm geometric tolerances to validate scanner accuracy under motion (articulation, speed variance).
- Color & Reflectance Calibration: Dual-spectrum (450–650 nm) validation across 24-color dental shade guides (VITA Classical & Bleached Shades).
- AI Feedback Loop: Calibration data trains on-device neural networks to correct motion blur, specular reflection, and gingival bleed artifacts.
3. Durability & Environmental Testing
To ensure clinical resilience, each scanner camera undergoes accelerated lifecycle testing simulating 5+ years of clinical use.
| Test Type | Protocol | Pass Criteria |
|---|---|---|
| Thermal Cycling | −10°C to +60°C over 1,000 cycles (IEC 60601-1-11) | No delamination, fogging, or signal drift >2% |
| Drop & Vibration | 1.2m drop (6 orientations); 5–500 Hz random vibration (IEC 6068-2-64) | Optical axis shift <1.2 arcmin; no solder fracture |
| Chemical Resistance | Exposure to 75% ethanol, glutaraldehyde, and saline mist (ISO 10993-23) | No lens coating degradation or sensor dark current increase >10% |
| Scan Cycle Endurance | 100,000+ on/off cycles with continuous streaming (20 fps) | Consistent SNR (>42 dB); no frame drop or latency spike |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the dominant force in high-performance, cost-optimized digital dentistry hardware. Carejoy Digital leverages this strategic advantage through:
- Integrated Supply Chain: Proximity to Tier-1 suppliers of CMOS sensors (e.g., GalaxyCore, Hua Day) and precision optics reduces lead times and logistics costs by up to 40%.
- Advanced Automation: Use of AI-guided robotic assembly lines reduces human error and increases throughput while maintaining ISO 13485 compliance.
- R&D Density: Shanghai and Shenzhen host over 60% of global dental imaging patent filings (2022–2025), enabling rapid innovation in open-architecture systems.
- Economies of Scale: High-volume production across multiple OEMs drives down BOM (Bill of Materials) costs without sacrificing quality.
- Regulatory Agility: NMPA fast-track approvals enable quicker market entry, which is mirrored in CE and FDA 510(k) pathways via harmonized standards.
As a result, Carejoy Digital delivers scanner systems with accuracy ≤ 8 µm RMS and open file support (STL/PLY/OBJ) at 30–50% below legacy European and North American equivalents—redefining the cost-performance frontier.
Tech Stack & Clinical Integration
- Open Architecture: Native export to STL, PLY, OBJ; seamless integration with exocad, 3Shape, and open-source CAD platforms.
- AI-Driven Scanning: Real-time intra-scan artifact correction using on-device CNN (Convolutional Neural Network).
- High-Precision Milling: Direct CAM export to Carejoy M400+ 5-axis mill (≤5 µm toolpath deviation).
- Cloud Sync & OTA Updates: Secure DICOM and patient data sync via Carejoy Cloud; remote firmware and AI model updates.
Support & Compliance
- 24/7 Remote Technical Support: Real-time diagnostics and screen-sharing via Carejoy Connect Portal.
- Software Updates: Monthly AI model refinements and security patches delivered over encrypted channels.
- Regulatory: ISO 13485:2016, CE MDR Class IIa, NMPA Class II, pending FDA 510(k).
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Scanner Camera.
✅ Open Architecture
Or WhatsApp: +86 15951276160
