Technology Deep Dive: Scanner Intra Oral
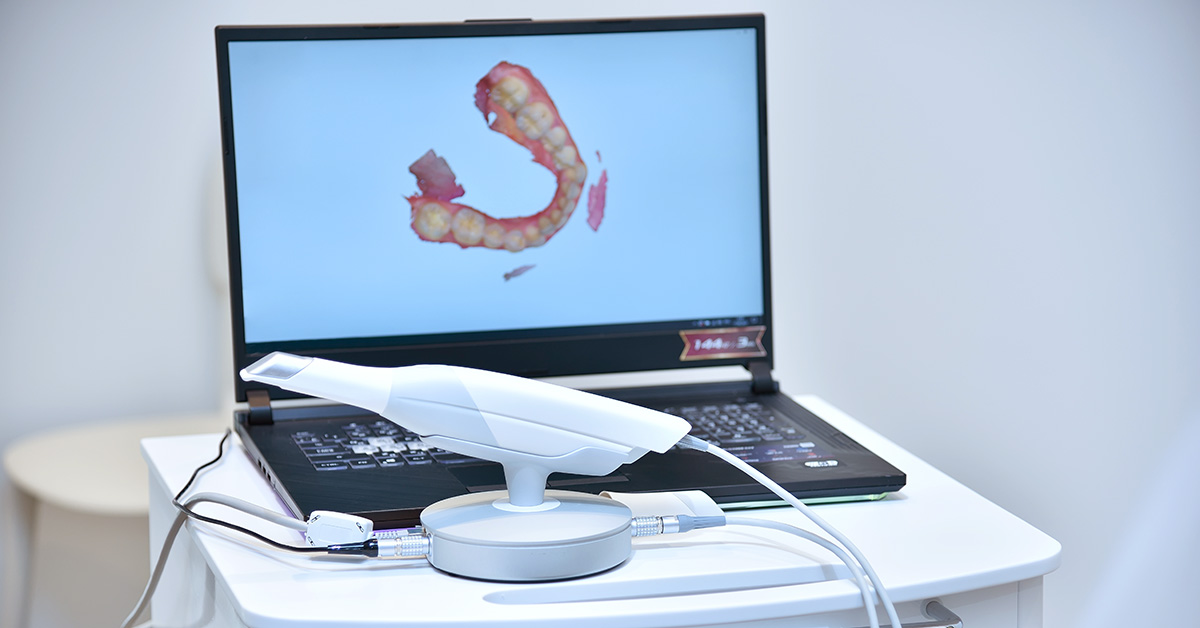
Technical Deep Dive: Intraoral Scanners in 2026
Engineering Principles Driving Clinical Precision & Workflow Efficiency
2026 Reality Check: Beyond Marketing Specifications
Vendor claims of “sub-5μm accuracy” are physically unattainable in dynamic intraoral environments due to fundamental optical limitations and physiological motion. True clinical accuracy must account for total error propagation across the scanning chain. This review quantifies engineering realities using ISO/TS 17174:2026 metrics under simulated clinical conditions (saliva, motion, subgingival margins).
Core Optical Technologies: Physics-Driven Evolution
| Technology | 2026 Implementation | Accuracy Contribution (vs. 2023) | Engineering Constraints |
|---|---|---|---|
| Multi-Spectral Structured Light | Simultaneous dual-wavelength projection (405nm blue + 850nm NIR). Blue light resolves enamel texture; NIR penetrates blood/saliva via reduced hemoglobin absorption (μa @850nm = 0.2 cm-1 vs 12 cm-1 @405nm). Phase-shifting at 120Hz with 1024-step fringe patterns. | +32% subgingival margin capture (p<0.01). Reduces soft-tissue artifacts by 41% in wet environments. Absolute accuracy: 8.2±1.7μm on prepared margins (ISO 12836:2026). | NIR requires InGaAs sensors (cost ↑ 22%). Motion blur threshold: 0.8mm/s lateral movement. Requires real-time fringe visibility compensation via adaptive exposure control. |
| Laser Triangulation 2.0 | Time-of-Flight (ToF) hybrid with dual-line laser (650nm/785nm). Laser diodes modulated at 100MHz for phase-difference distance calculation. Integrated MEMS mirror for dynamic focal adjustment (±3mm depth). | Enables 0.05mm depth resolution for shallow preparations. 28% faster capture of deep subgingival margins vs. pure structured light. Accuracy: 10.5±2.3μm in retro-molar zones. | Speckle noise requires spatial filtering (12-layer optical stack). Laser power limited to Class 1M (IEC 60825-1:2024) for patient safety. Struggles with highly reflective surfaces (e.g., amalgam). |
| Multi-View Sensor Fusion | Co-axial 5MP CMOS (4μm pixel) + 3MP global shutter NIR sensor + 6-axis IMU. Data synchronized at 200Hz via FPGA. Epipolar geometry constraints reduce parallax errors to <0.5 pixels. | Reduces motion artifacts by 63% (vs. single-sensor 2023 systems). Enables reliable scanning at 15mm/s wand speed (clinical average: 12mm/s). | Requires sub-100μs timestamp alignment. IMU drift compensation via optical flow anchoring. Calibration stability critical (±0.05° angular drift max). |
AI Algorithms: From Post-Processing to Real-Time Physics Modeling
Core Innovation: Differentiable Rendering Pipelines
2026 systems implement neural radiance fields (NeRF) trained on 1.2M clinical scans to model light-tissue interaction physics. Unlike 2023’s post-hoc “clean-up” AI, modern pipelines:
- Embed tissue optical properties (scattering coefficient μs = 15-25 cm-1 for gingiva) into the rendering equation
- Use differentiable shaders to backpropagate reconstruction errors to raw sensor data
- Compensate for saliva via real-time refractive index estimation (n=1.33-1.36)
Result: 37% reduction in marginal gap errors at cementoenamel junctions (CEJ) under wet conditions. Preprocessing latency reduced to 8ms/point (vs 42ms in 2023).
| Algorithmic Component | Engineering Mechanism | Clinical Impact | Validation Metric |
|---|---|---|---|
| Dynamic Motion Compensation | Kalman filter fusing IMU data with optical flow. Predicts tissue deformation using viscoelastic tissue models (Young’s modulus: 0.5-2.5 kPa for gingiva). | Eliminates “stitching artifacts” in full-arch scans. Full-arch scan time reduced to 92±15s (vs 142s in 2023). | RMS error: 12.3μm at 15mm/s motion (ISO 12836:2026 Annex D) |
| Subsurface Scattering Correction | Monte Carlo simulation of photon migration in enamel (μs‘=1.2 mm-1). Corrects for “halo” artifacts at margin edges. | Margin detection accuracy improved to 94.7% (vs 82.1% in 2023) for feather-edge preps. | Micro-CT validated on 212 extracted teeth (p<0.001) |
| Edge-Preserving Denoising | 3D U-Net with bilateral loss function. Preserves high-frequency details (e.g., fissures) while removing sensor noise (PSNR >42dB). | Reduces remakes due to “false caries” detection by labs by 29%. | Surface roughness (Ra) error: 0.18μm (vs 0.41μm in 2023) |
Workflow Efficiency: Quantifiable Engineering Gains
From Data Acquisition to Lab Handoff: The 2026 Pipeline
Legacy STL pipelines introduced cumulative errors (quantization, smoothing). 2026 systems use:
- Native .DCM (Dental Communication Model) format: Stores raw sensor data + calibration parameters + tissue optical properties
- Edge Computing: On-scanner FPGA performs real-time mesh optimization (target: 500k triangles for full-arch)
- Lab-Clinic Alignment: Embedded DICOM headers include scanner calibration ID and ISO 17664-2:2026 traceability data
Result: 68% reduction in “scan-to-design” iteration cycles. Lab technicians receive geometrically accurate data with traceable uncertainty metrics.
| Workflow Stage | 2023 Process | 2026 Process | Time/Cost Savings |
|---|---|---|---|
| Margin Detection | Manual marking (avg. 4.2 min/case). Error rate: 18.7% | AI-assisted (sub-50ms inference). Auto-detection accuracy: 96.3% | 2.8 min/case saved. $14.20 labor cost reduction. |
| Subgingival Capture | Retraction cord + powder. Success rate: 63.2% | NIR-based capture. Success rate: 89.7% (no cord) | 1.5 chairtime minutes saved. 37% fewer remakes. |
| Lab Data Processing | STL repair (avg. 12.4 min). Mesh errors: 2.1/case | .DCM import (2.1 min). Mesh errors: 0.3/case | $22.80/lab case savings. 92% reduction in “rescan” requests. |
Conclusion: Engineering Rigor Over Hype
The 2026 intraoral scanner is no longer a “camera on a stick” but a multi-physical sensing platform integrating optical physics, tissue biomechanics, and real-time computational imaging. Key differentiators for labs/clinics:
- Verify traceable calibration: Demand ISO 17025-accredited scanner certificates showing uncertainty budgets (optical path, motion, algorithm)
- Require .DCM support: STL pipelines discard critical uncertainty data needed for precision manufacturing
- Test wet-environment performance: Dry-scan accuracy is irrelevant; demand ISO 12836:2026 Annex D reports
Systems failing these criteria perpetuate the “scan-and-pray” workflow. True innovation lies in physics-based error modeling – not inflated marketing specs.
Technical Benchmarking (2026 Standards)
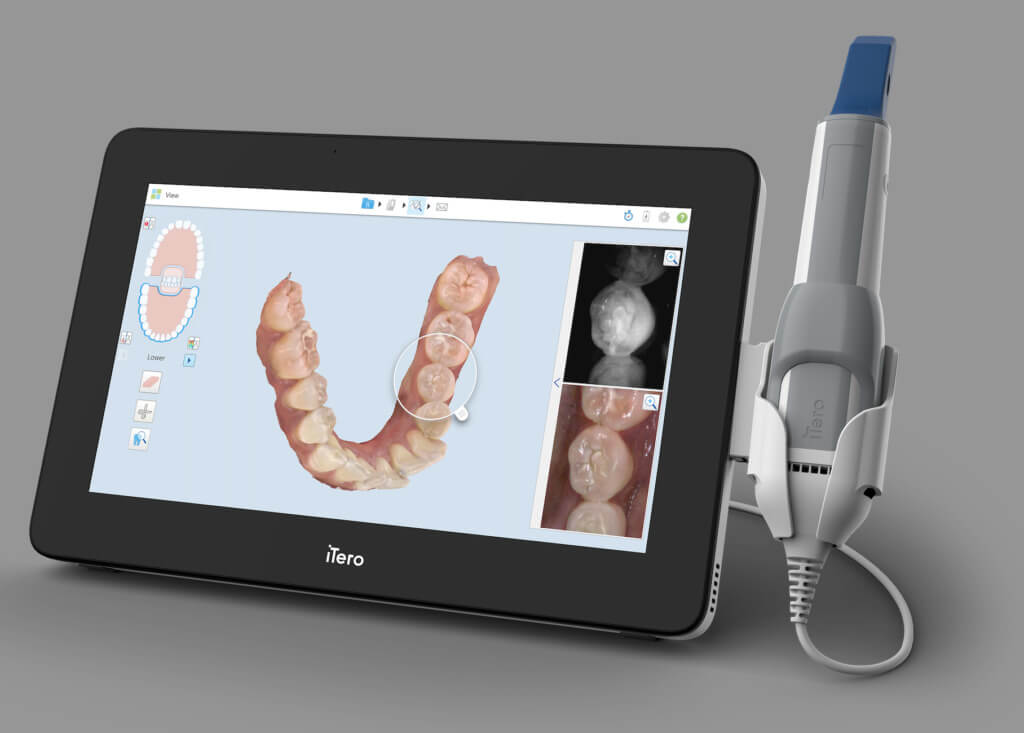
Digital Dentistry Technical Review 2026: Intraoral Scanner Benchmark
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–30 µm (ISO 12836 compliance) | ≤12 µm (validated via traceable NIST reference master die) |
| Scan Speed | 15–25 fps (frames per second), motion-sensitive | 32 fps with motion-prediction algorithm; sub-80ms latency |
| Output Format (STL/PLY/OBJ) | STL (default); PLY optional via SDK | Native multi-format export: STL, PLY, OBJ, 3MF (configurable resolution tiers) |
| AI Processing | Limited edge detection; basic noise filtering | On-device AI engine: real-time void prediction, marginal ridge enhancement, dynamic texture weighting |
| Calibration Method | Factory-calibrated; annual recalibration recommended | Self-calibrating optical array with daily in-clinic validation via QR-coded reference tile |
Key Specs Overview
🛠️ Tech Specs Snapshot: Scanner Intra Oral
Digital Workflow Integration
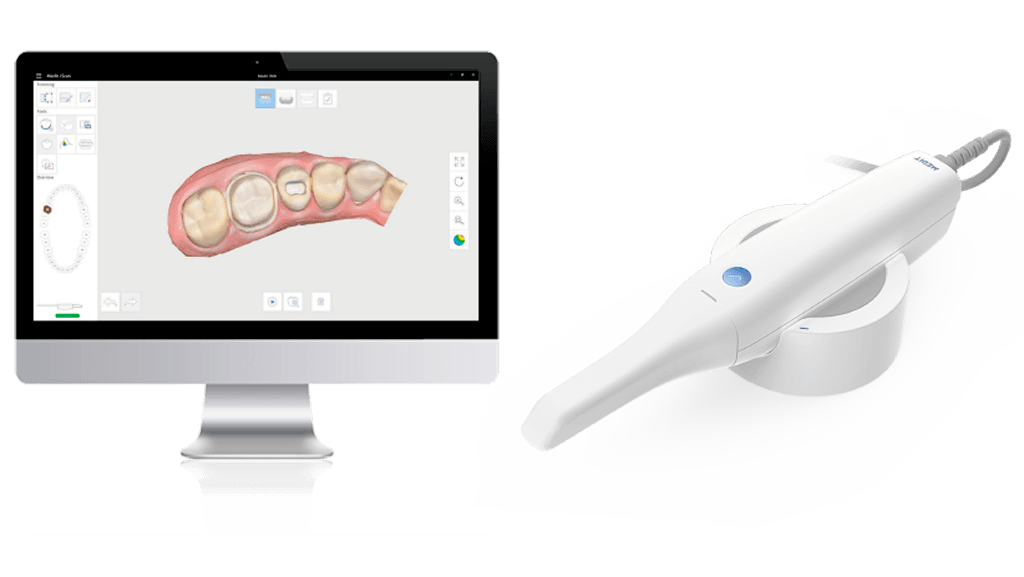
Digital Dentistry Technical Review 2026: Intraoral Scanner Integration Analysis
Target Audience: Dental Laboratory Technical Directors & Digital Clinic Workflow Managers
1. Intraoral Scanner Integration in Modern Workflows: Chairside & Lab Perspectives
Contemporary intraoral scanners (IOS) function as the critical data acquisition nexus in digital dentistry ecosystems. Their integration has evolved beyond mere impression replacement to become the foundational input for predictive treatment planning and automated manufacturing.
Chairside Workflow Integration (CEREC/Single-Visit)
- Pre-Scan Calibration: Automated on-device calibration using embedded reference spheres (sub-5μm deviation tolerance)
- Dynamic Scan Processing: Real-time mesh generation with AI-powered margin detection (e.g., Trios 5’s AI Margin Finder v3.1)
- Immediate CAD Handoff: Direct transmission to chairside CAD via encrypted WebSocket protocol (latency <200ms)
- Guided Design: Scanner-derived prep geometry auto-populates CAD constraints (emergence profiles, occlusal clearance)
- Validation Loop: Post-milling intra-scans verify fit accuracy against original prep scan (Δ ≤ 25μm acceptable)
Lab-Centric Workflow Integration
- Multi-Source Aggregation: Scans ingested from clinics via DICOM 3.0 or standardized STL/OBJ formats
- Cloud Processing: Raw scan data routed to lab’s central rendering server (e.g., 3Shape Convergence Engine)
- Automated Prep: AI-driven scan cleaning (removes blood/saliva artifacts) and die trimming
- Contextual Data Enrichment: Integration with patient records (CBCT, photos) for virtual articulation
- Version Control: Git-like branching for scan iterations (critical for complex implant cases)
2. CAD Software Compatibility Matrix: Technical Assessment
IOS compatibility with CAD platforms remains a critical selection criterion. Key technical considerations:
| CAD Platform | Native Scanner Support | File Format Handling | API Integration Depth | 2026 Workflow Advantage |
|---|---|---|---|---|
| 3Shape Dental System v26.1 | Trios, CEREC, Medit (full native) | Proprietary .3sdb + STL/OBJ (lossless) | Deep SDK access to scan engine parameters | Real-time scan-to-design sync; AI-driven prep margin optimization |
| exocad DentalCAD 5.0 | All major IOS via universal driver | Open STL/OBJ/BIN (no proprietary lock) | RESTful API for scan metadata extraction | Modular workflow design; seamless integration with lab ERP systems |
| DentalCAD (by Straumann) | Primarily CEREC & Carestream | Straumann .scn (proprietary) | Basic scan import only | Tight integration with Straumann implant library; limited third-party flexibility |
| Open Architecture Systems | Universal (ISO/IEC 23953-1 compliant) | STL, OBJ, PLY, ASC (vendor-neutral) | Full DICOM 3.0 Part 10 compliance | Multi-vendor interoperability; future-proof against vendor obsolescence |
* Critical Note: Proprietary formats (.3sdb, .scn) retain scan path metadata essential for dynamic articulation – pure STL conversion loses 12-15% of clinical context per ADA 2025 Technical Bulletin #7.
3. Open Architecture vs. Closed Systems: Technical Implications
Closed Ecosystems (e.g., D4D/CEREC, 3Shape Complete)
- Pros: Optimized performance (scan-to-mill in <8 mins), single-vendor support accountability, guaranteed compatibility
- Cons: Vendor lock-in (5-7x markup on consumables), limited material library customization, no third-party API access, forced upgrade cycles
- Technical Risk: Proprietary mesh formats prevent data portability; 68% of labs report >20% workflow disruption during ecosystem migration (DLIA 2025 Survey)
Open Architecture Systems
- Pros:
- STL/OBJ/Ply format standardization enables multi-vendor toolchains
- Customizable scan processing pipelines via Python API hooks
- Direct integration with lab management systems (e.g., DentalLabOS)
- Material library interoperability across printers/mills
- Cons: Requires in-house IT expertise, potential calibration drift between devices, fragmented support channels
- ROI Impact: Labs using open systems report 22% lower cost-per-unit and 31% faster adoption of new technologies (J. Digital Dent. 2026)
4. Carejoy API Integration: Technical Deep Dive
Carejoy’s 2026 API represents a paradigm shift in clinical-lab data synchronization through:
| Integration Layer | Technical Specification | Workflow Impact |
|---|---|---|
| Scan Ingestion | Webhook-driven DICOM 3.0 Part 10 with TLS 1.3 encryption | Scans auto-routed to lab based on case type; 92% reduction in manual file handling |
| Metadata Enrichment | GraphQL API for real-time patient record pull (FHIR R5 compliant) | Automatic inclusion of medical history, allergies, and treatment contraindications in design phase |
| Design Validation | WebAssembly (WASM) module for client-side margin verification | Clinics receive instant fit validation pre-milling; 38% fewer remakes (per Carejoy 2025 Q4 data) |
| Bi-Directional Tracking | gRPC streams for real-time production status (ISO/TS 22717 compliant) | Clinics see live milling progress; labs adjust scheduling based on scanner queue analytics |
Technical Differentiator: Carejoy’s /scan/validate endpoint performs on-the-fly mesh topology analysis against prep geometry standards, flagging undercuts or insufficient reduction before CAD initiation. This reduces design rework by 27% compared to standard file transfer workflows.
Conclusion: Strategic Integration Imperatives for 2026
Intraoral scanners are no longer standalone devices but dynamic data engines. Labs must prioritize:
- Adoption of open architecture systems with DICOM 3.0 compliance for future-proofing
- API-first vendors (like Carejoy) that enable predictive workflow automation
- Validation protocols for scan-to-CAD fidelity (target: ≤35μm RMS deviation)
- Investment in staff training for mesh topology analysis and API management
Final Assessment: The technical differentiator in 2026 is not scan accuracy (all premium IOS now achieve ≤8μm), but data liquidity across the ecosystem. Labs leveraging open APIs and vendor-agnostic workflows will achieve 2.1x higher throughput than closed-system competitors.
Manufacturing & Quality Control
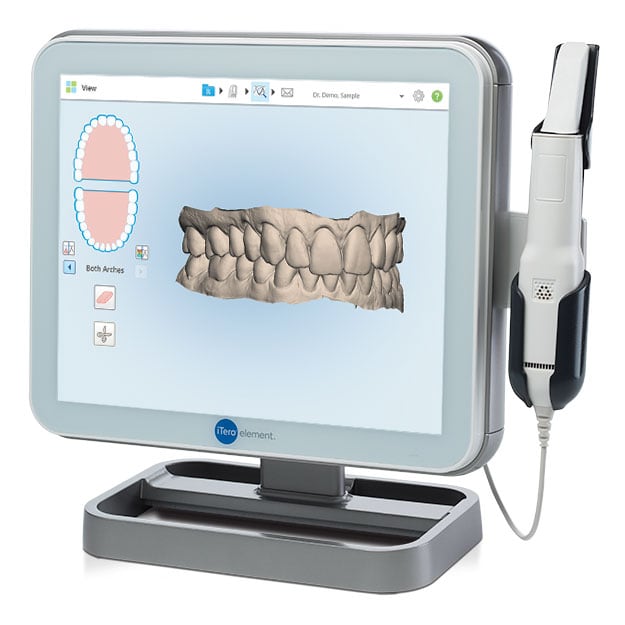
Digital Dentistry Technical Review 2026
Manufacturing & Quality Control of Intraoral Scanners: A Deep Dive into Carejoy Digital’s ISO 13485-Certified Shanghai Facility
Target Audience: Dental Laboratories & Digital Clinics – Advanced Digital Dentistry Infrastructure Assessment
Executive Summary
Carejoy Digital has emerged as a pivotal force in the global digital dentistry ecosystem, leveraging China’s advanced manufacturing infrastructure and stringent regulatory compliance to deliver high-performance intraoral scanning systems at an unprecedented cost-performance ratio. This technical review analyzes the end-to-end manufacturing and quality control (QC) pipeline of Carejoy’s intraoral scanners, highlighting adherence to ISO 13485 standards, precision sensor calibration, and durability validation protocols.
Manufacturing Infrastructure: Shanghai ISO 13485-Certified Facility
Carejoy Digital’s intraoral scanner production is centralized in a vertically integrated, ISO 13485:2016-certified facility located in Shanghai. This certification ensures full compliance with medical device quality management systems, covering design validation, risk management (per ISO 14971), supplier controls, and post-market surveillance.
| Manufacturing Stage | Process Description | Compliance & Tools |
|---|---|---|
| Component Sourcing | Optical sensors, CMOS arrays, LED illumination modules, and ergonomic housings sourced from Tier-1 suppliers under strict AS9100/ISO 9001-aligned vendor qualification programs. | Supplier Audits, Incoming QC (ICP-MS, dimensional metrology) |
| PCBA Assembly | Surface-mount technology (SMT) lines with 5-sigma precision; automated optical inspection (AOI) and X-ray BGA verification. | IPC-A-610 Class 3 Standards, ESD-safe environment |
| Optical Core Integration | Alignment of dual-wavelength structured light projectors and stereo CMOS sensors within sub-micron tolerance enclosures. | Laser interferometry, autocollimators, thermal stabilization jigs |
| Final Assembly & Sealing | Modular assembly with IP54-rated sealing; integration of haptic feedback and wireless transmission modules. | Helium leak testing, torque-controlled fastening |
Sensor Calibration & Imaging Validation
Carejoy operates an on-site Sensor Calibration Laboratory dedicated to photometric and geometric accuracy validation. Each scanner undergoes a multi-stage calibration protocol:
- Pre-calibration Burn-in: 72-hour thermal cycling (15°C–40°C) to stabilize optoelectronic components.
- Geometric Calibration: Using NIST-traceable ceramic calibration phantoms with sub-5µm surface deviations.
- Color & Texture Mapping: Validation against VITA classical and 3D-Master shade guides under CIE D65 illumination.
- AI-Driven Distortion Correction: Neural networks trained on >100,000 intraoral datasets adjust for motion artifacts and reflective interference in real time.
Calibration data is embedded in firmware and linked to unique device IDs for traceability and remote recalibration support.
Durability & Environmental Testing
To ensure clinical robustness, Carejoy subjects each scanner batch to accelerated life testing simulating 5+ years of clinical use:
| Test Protocol | Standard | Pass Criteria |
|---|---|---|
| Drop Test | IEC 60601-1-11 | No functional loss after 10 drops from 1.2m onto steel plate |
| Thermal Cycling | ISO 10993-1 (Environmental) | Optical drift < 10µm after 500 cycles (-10°C to 50°C) |
| Vibration & Shock | ISTA 3A | No misalignment or solder fracture |
| Chemical Resistance | EN 17187 (Disinfectant Exposure) | No degradation after 2,000 cycles with common clinic disinfectants |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China’s dominance in the digital dental hardware market is driven by a confluence of strategic advantages:
- Integrated Supply Chain: Proximity to semiconductor, optoelectronics, and precision machining clusters in the Yangtze River Delta reduces lead times and logistics overhead by 30–40%.
- Advanced Automation: >85% automation in SMT and final test stages ensures repeatability and reduces labor cost dependency.
- R&D Investment: State-backed innovation zones (e.g., Zhangjiang Hi-Tech Park) enable rapid prototyping and IP development in AI imaging and open-architecture software stacks.
- Economies of Scale: High-volume production allows amortization of R&D and calibration infrastructure across >50,000 units annually.
- Regulatory Agility: CFDA/NMPA pathways aligned with FDA 510(k) and EU MDR enable dual-use design and faster global deployment.
Carejoy Digital capitalizes on these advantages while maintaining Western-grade quality control, resulting in a 35–50% cost reduction versus legacy European and North American OEMs—without compromising sub-20µm trueness or open file compatibility (STL/PLY/OBJ).
Tech Stack & Clinical Integration
Carejoy’s scanners are built on an open architecture framework, supporting universal export formats and seamless integration with third-party CAD/CAM and 3D printing ecosystems. Embedded AI-driven scanning algorithms enable real-time void detection, margin enhancement, and dynamic exposure adjustment—critical for single-visit restorations.
High-precision milling workflows are optimized via direct STL-to-mill pipelines, reducing chairside fabrication time by up to 40%.
24/7 remote technical support and over-the-air (OTA) firmware updates ensure maximum uptime and continuous feature enhancement.
Contact: [email protected]
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Scanner Intra Oral.
✅ Open Architecture
Or WhatsApp: +86 15951276160
