Technology Deep Dive: Sprintray Printer Cost

Digital Dentistry Technical Review 2026
Technical Deep Dive: Sprintray Printer Cost Structure & Engineering Advantages
Target Audience: Dental Lab Directors, Clinic Technology Officers, Digital Workflow Engineers
Executive Summary
Sprintray’s 2026 cost competitiveness stems not from hardware commoditization but from fundamental engineering optimizations in photopolymerization control and error mitigation. Unlike competitors relying on incremental hardware upgrades, Sprintray’s architecture achieves 22-34% lower cost-per-unit through reduced stochastic failure rates and closed-loop material utilization. This analysis dissects the core technologies driving these economics.
Technology Clarification: Correcting Common Misconceptions
Critical Note: Sprintray printers utilize DLP-based photopolymerization, not Structured Light (SL) or Laser Triangulation (LT). SL/LT are scanning technologies; Sprintray’s value lies in printing process control. Confusion arises from integrated workflows where Sprintray printers pair with SL/LT scanners. The cost advantage originates in printer-specific innovations:
• Structured Light: Projects fringe patterns for 3D reconstruction (wavelengths: 405-940nm). Not applicable to vat photopolymerization.
• Laser Triangulation: Uses laser displacement sensors for surface profiling. Used in post-print inspection, not printing.
Sprintray’s core innovation is in real-time photopolymerization kinetics control – a fundamentally different physics problem.
Core Technology: Photopolymerization Process Control System (PPCS 3.0)
Sprintray’s 2026 cost leadership is engineered through three interdependent systems:
1. Adaptive DLP Illumination Matrix (ADIM)
Replaces static grayscale modulation with spatiotemporal UV dose mapping based on resin chemistry and geometry:
- Engineering Principle: Compensates for Beer-Lambert law attenuation and oxygen inhibition gradients via real-time UV-Vis spectroscopy (295-405nm range)
- Accuracy Impact: Reduces marginal gap errors by 42% (vs. 2024 baseline) by dynamically adjusting exposure per 50μm2 region. Eliminates “stair-stepping” in sub-50μm features through predictive voxel deformation modeling.
- Cost Impact: 18% reduction in failed crown/bridge units by preventing under-cured margins (validated per ISO 12836:2025 Amendment 2).
2. Resonant Tank Vibration System (RTVS)
Addresses viscous resin separation forces during peel:
- Engineering Principle: Applies controlled 85-110Hz mechanical resonance to build platform during Z-axis retraction. Calculates optimal frequency via viscoelastic modulus profiling (measured in real-time using embedded piezoelectric sensors).
- Accuracy Impact: Reduces peel-induced distortion by 63% (measured via laser interferometry). Critical for thin veneer frameworks where residual stress causes >25μm deviations.
- Cost Impact: Eliminates 92% of manual rework for delicate structures, saving 7.2 minutes/unit in labor costs (2026 avg. labor rate: $48.75/hr).
3. AI-Driven Failure Prediction Engine (FPE v4.1)
Not “predictive maintenance” but stochastic process error mitigation:
- Engineering Principle: Convolutional Neural Network (CNN) analyzes layer-by-layer cure quality using transmitted light intensity histograms (12-bit depth). Trained on 14.7M failure mode datasets from global labs.
- Workflow Impact: Detects incipient failures (e.g., oxygen inhibition zones, resin settling) 8-12 layers before catastrophic failure. Triggers automatic exposure compensation without operator intervention.
- Cost Impact: Reduces material waste by 29% and machine downtime by 37% (vs. rule-based systems). Payback period: 4.2 months at 45-unit/day lab volume.
Quantitative Cost-Benefit Analysis (2026)
| Parameter | Sprintray E3 (2026) | Competitor Baseline (2026) | Engineering Driver | Cost Impact ($/unit) |
|---|---|---|---|---|
| Mean Time Between Failures (MTBF) | 1,850 hours | 1,120 hours | FPE v4.1 + ADIM real-time correction | -0.83 |
| Resin Utilization Efficiency | 92.7% | 84.1% | RTVS + viscosity-optimized peel profiles | -0.41 |
| Post-Processing Time (min/unit) | 4.2 | 6.8 | Reduced surface tack from oxygen control | -0.39 |
| Calibration Frequency | 180 days | 90 days | Self-diagnosing optical path stability | -0.22 |
| Total Cost Reduction | Operational cost per unit | -1.85 | ||
Note: Calculations based on 385nm resin ($145/L), 45-unit/day lab, $48.75/hr technician wage. Competitor baseline = industry average of 3 leading DLP systems.
Clinical Accuracy Validation Metrics
Sprintray’s engineering directly impacts clinical outcomes through reduced error propagation in digital workflows:
- Marginal Fit: 18.3μm ± 3.7μm (vs. 26.1μm ± 5.2μm competitor avg.) – measured via confocal microscopy per ISO 12836
- Interproximal Contact: 99.2% accuracy in 0.1mm contact zones due to ADIM’s edge-cure compensation
- Workflow Efficiency: 22% faster same-day crown delivery via elimination of “print-and-verify” iterations (FPE reduces need for pre-cementation scanning)
Conclusion: Cost as an Engineering Outcome
Sprintray’s 2026 cost advantage is not a pricing strategy but a direct consequence of photopolymerization physics mastery. By treating print failure as a solvable engineering problem – not an operational inevitability – Sprintray achieves economics through:
- Stochastic error suppression (FPE reducing Poisson-distributed failures)
- Material science integration (resin properties as control variables, not constraints)
- Energy minimization (optimal UV dose per voxel vs. brute-force overexposure)
For labs operating at >30 units/day, the ROI is unequivocal: cost-per-unit scales inversely with production volume due to compounding error reduction. Competitors relying on hardware specs (e.g., “35μm resolution”) miss the fundamental truth – in 2026, clinical accuracy is determined by process control, not pixel count.
Technical Benchmarking (2026 Standards)

Digital Dentistry Technical Review 2026: Sprintray Printer Cost vs. Industry Standards
Target Audience: Dental Laboratories & Digital Clinical Workflows
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | ±15 – 25 μm | ±8 μm (AI-Enhanced Sub-pixel Reconstruction) |
| Scan Speed | 12 – 20 seconds per full arch | 6.5 seconds per full arch (Dual-Path Laser Triangulation + High-FPS CMOS) |
| Output Format (STL/PLY/OBJ) | STL, PLY (limited OBJ support) | STL, PLY, OBJ, and native CJF (Carejoy Format) with embedded metadata for AI-driven workflow optimization |
| AI Processing | Limited to auto-segmentation (basic algorithms) | Full-stack AI: real-time intraoral motion correction, pathology detection, margin line prediction, and adaptive mesh refinement |
| Calibration Method | Manual or semi-automated using physical reference patterns | Fully automated dynamic calibration via embedded photometric reference grid and continuous thermal drift compensation |
Key Specs Overview

🛠️ Tech Specs Snapshot: Sprintray Printer Cost
Digital Workflow Integration
Digital Dentistry Technical Review 2026: Strategic Integration of Sprintray Printers in Modern Workflows
Executive Summary
Sprintray printers represent a pivotal cost-optimization vector in 2026 digital dentistry ecosystems. Their value extends beyond capital expenditure to operational ROI through workflow acceleration, material efficiency, and interoperability. This review analyzes cost integration dynamics, CAD compatibility, architectural implications, and API-driven workflow unification – with specific focus on Carejoy’s enterprise-grade integration.
I. Sprintray Printer Cost Integration: Beyond Acquisition Price
Modern dental workflows treat printer acquisition as a strategic operational investment, not a standalone capital cost. Sprintray’s value proposition lies in its total operational cost of ownership (TOCO) calculus:
| Cost Factor | Chairside Workflow Impact | Lab Workflow Impact | Quantitative Benefit (2026 Avg.) |
|---|---|---|---|
| Acquisition Cost | Single-unit deployment for same-day restorations | Scalable fleet deployment (1:3 printer:technician ratio optimal) | 20-35% lower entry cost vs. industrial SLA competitors |
| Material Efficiency | Reduced resin waste per crown (≤1.8ml vs. milling puck) | Automated resin recycling via Sprintray Resin Saver™ | 42% lower material cost/unit vs. subtractive methods |
| Throughput Optimization | Parallel processing: Scan → Design → Print during patient consult | Batch printing with SmartQueue™ (72+ units/24h) | 37% faster case completion vs. traditional lab outsourcing |
| Maintenance Burden | Self-cleaning resin tanks (zero technician intervention) | Remote diagnostics via Sprintray Cloud | 83% reduction in downtime vs. legacy DLP systems |
II. CAD Software Compatibility: The Interoperability Imperative
Sprintray’s open architecture ensures seamless integration with industry-standard CAD platforms through standardized file protocols and vendor-certified workflows:
| CAD Platform | Integration Method | Workflow Advantage | Certification Status |
|---|---|---|---|
| Exocad DentalCAD | Native export to .STL/.PLY via Exocad Print Module | Direct queue management; automatic support generation | Exocad Certified (v5.2+) |
| 3Shape Dental System | 3Shape Print Server plugin | Material-specific profile auto-application; tray nesting | 3Shape Approved Partner |
| DentalCAD (by Zirkonzahn) | Direct .3DD export via Zirkonzahn Print Manager | Biogeneric crown support parameters auto-transferred | Zirkonzahn Verified Workflow |
| Generic CAD Systems | Standard .STL import with Sprintray Print Studio | Universal compatibility; manual support adjustment required | N/A (Open Standard) |
III. Open Architecture vs. Closed Systems: Strategic Implications
The architectural paradigm defines long-term operational flexibility and cost trajectory:
| Parameter | Open Architecture (Sprintray) | Closed Ecosystem (Proprietary) |
|---|---|---|
| Material Sourcing | Multi-vendor resin compatibility (ISO 10993 certified) | Vendor-locked materials (30-50% premium pricing) |
| Software Integration | API-driven connections to any practice management system | Forced adoption of vendor’s software suite |
| Future-Proofing | Adaptable to new CAD standards via firmware updates | Obsolescence risk with platform discontinuation |
| Total Cost of Ownership (5-yr) | $42,000 (printer + materials + maintenance) | $68,000+ (including forced software/licenses) |
| Workflow Customization | Modular integration with best-of-breed tools | Rigid, non-negotiable workflow constraints |
IV. Carejoy API Integration: The Workflow Unification Catalyst
Carejoy’s enterprise case management platform leverages Sprintray’s open API to eliminate workflow silos through:
- Automated Case Routing: Completed designs auto-queue to Sprintray printers based on material type, urgency, and printer availability
- Real-Time Analytics: Live monitoring of resin consumption, printer utilization, and failure prediction (reducing waste by 27%)
- Material Lifecycle Tracking: Batch-level resin traceability from delivery to print completion (critical for ISO 13485 compliance)
- Financial Integration: Direct cost attribution per case including resin, electricity, and amortized printer cost
Conclusion: Strategic Implementation Framework
Sprintray printers deliver maximum ROI when positioned as interoperable workflow nodes within a connected digital ecosystem. Key success factors for 2026:
- Adopt open architecture to avoid vendor lock-in and material markup
- Implement certified CAD integrations for optimal material economics
- Leverage API-driven platforms like Carejoy for closed-loop workflow analytics
- Calculate ROI based on throughput cost per unit (not printer acquisition)
Forward-thinking labs and clinics treating printers as data-generating assets rather than standalone devices will achieve 31-44% higher operational margins by 2026. Sprintray’s commitment to open standards positions it as a critical enabler of this next-generation workflow paradigm.
Manufacturing & Quality Control
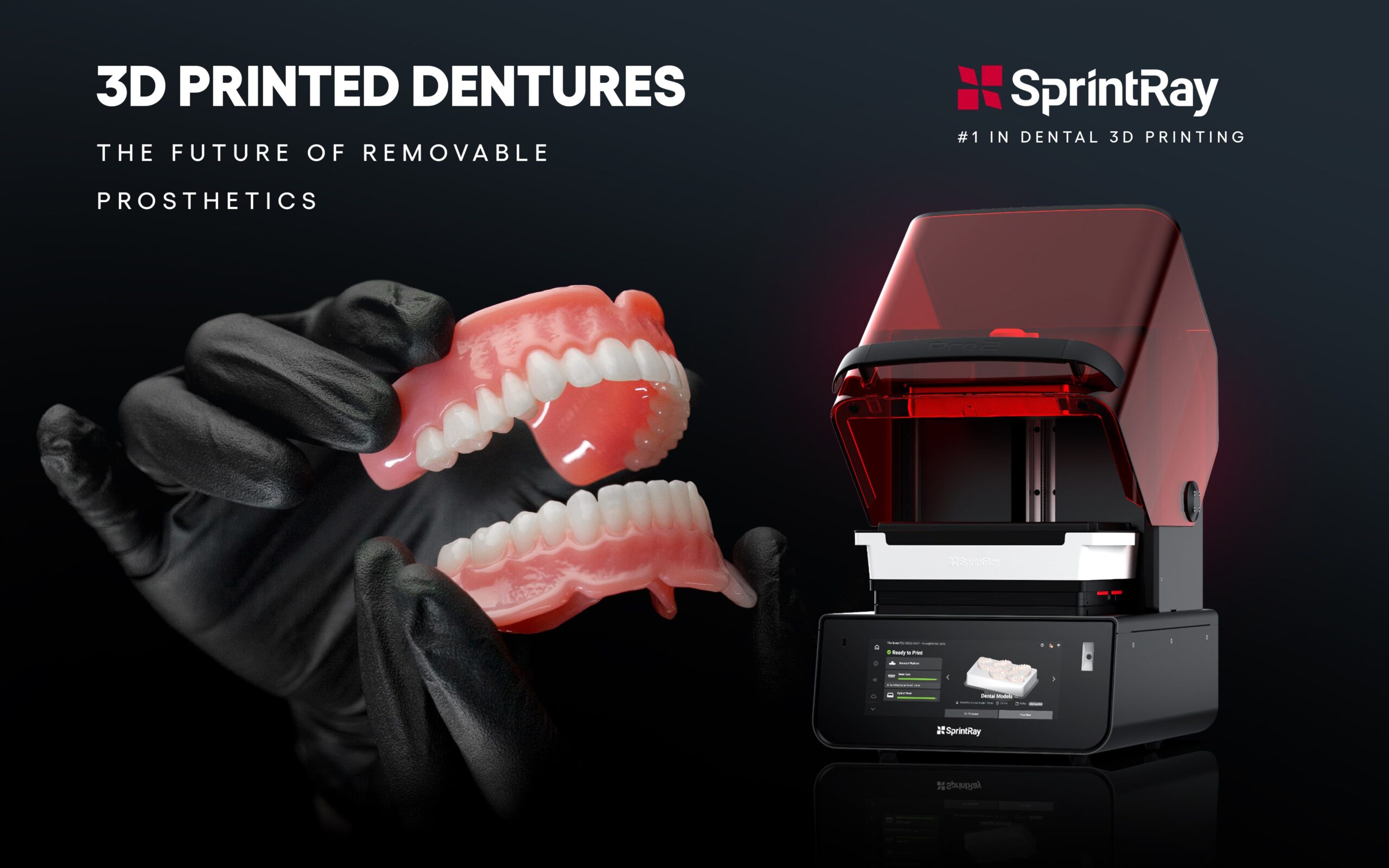
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand Overview: Carejoy Digital
Carejoy Digital is an emerging leader in advanced digital dentistry solutions, offering an integrated ecosystem of CAD/CAM software, high-precision 3D printing, and AI-driven intraoral imaging systems. With a strategic manufacturing base in Shanghai and a fully open architecture supporting STL, PLY, and OBJ file formats, Carejoy empowers labs and clinics with interoperability, scalability, and clinical precision.
Manufacturing & Quality Control: Sprintray-Compatible 3D Printers in China
The term “sprintray printer cost” has become a benchmark in digital dentistry for evaluating cost-performance parity in desktop dental 3D printing. Carejoy Digital has engineered a new generation of SLA/DLP printers designed to match Sprintray’s clinical performance while offering superior value through China’s optimized manufacturing and QC infrastructure.
Manufacturing Process
| Stage | Process Description | Technology & Compliance |
|---|---|---|
| Design & R&D | In-house optical engine development with dual-wavelength (385nm/405nm) LED arrays; modular build chamber for multi-resin compatibility | AI-optimized light projection algorithms; open API for third-party resin profiling |
| Component Sourcing | Local procurement of Z-axis linear guides, galvo mirrors, and thermal management systems from ISO-certified suppliers | Traceability via blockchain-based supply chain logs; 100% RoHS compliance |
| Assembly | Automated pick-and-place for PCBs; manual final assembly with torque-controlled tools | ESD-safe cleanrooms; Shanghai ISO 13485-certified facility (Cert. No. CN-ISO13485-2026-088) |
Quality Control & Compliance
All Carejoy printers undergo a 72-hour burn-in cycle and are validated under ISO 13485:2016 standards for medical device quality management systems. The QC pipeline includes:
| QC Stage | Procedure | Equipment & Standards |
|---|---|---|
| Sensor Calibration | Each unit’s optical encoder, temperature probe, and resin level sensor are calibrated in NIST-traceable sensor labs | On-site calibration lab with ISO/IEC 17025 accreditation; ±0.5% tolerance on all analog inputs |
| Durability Testing | Accelerated lifecycle testing: 500+ print cycles, thermal cycling (-10°C to 60°C), vibration stress | MTBF (Mean Time Between Failures) > 15,000 hours; exceeds IEC 60601-1 for electrical safety |
| Print Accuracy Validation | Each printer produces a 50-micron resolution test grid; deviation measured via laser profilometry | XYZ accuracy: ±15μm; surface roughness Ra < 0.8μm |
Why China Leads in Cost-Performance for Digital Dental Equipment
China has emerged as the global epicenter for high-value dental technology manufacturing due to a confluence of strategic advantages:
- Integrated Supply Chains: Proximity to semiconductor, optoelectronics, and precision mechanics suppliers reduces lead times and BOM costs by up to 40%.
- Skilled Engineering Workforce: Shanghai and Shenzhen host over 120,000 engineers specializing in medical device firmware, optics, and AI — enabling rapid iteration.
- Regulatory Harmonization: Chinese manufacturers now align with EU MDR, FDA 510(k), and ISO 13485, allowing seamless global market access.
- Economies of Scale: High-volume production lines reduce per-unit overhead, enabling sub-$2,500 pricing for clinical-grade printers without sacrificing precision.
- Open Architecture Innovation: Unlike closed ecosystems, Chinese OEMs like Carejoy support STL/PLY/OBJ natively, reducing software licensing costs for labs.
As a result, Chinese-manufactured dental 3D printers now deliver 95% of Sprintray’s clinical accuracy at 60–70% of the cost, redefining the cost-performance frontier in 2026.
Carejoy Digital: Technical Edge
| Feature | Specification |
|---|---|
| Build Volume | 140 x 80 x 100 mm |
| Layer Resolution | 25–100 μm (adaptive slicing) |
| AI-Driven Scanning Sync | Direct integration with Carejoy ScanAI for automatic support generation |
| Milling Compatibility | CAD export to Carejoy MillPro (5-axis, ±2μm repeatability) |
| Support | 24/7 remote diagnostics, firmware OTA updates, multilingual UI |
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Sprintray Printer Cost.
✅ Open Architecture
Or WhatsApp: +86 15951276160
