Technology Deep Dive: Straumann Intraoral Scanner

Digital Dentistry Technical Review 2026: Straumann Intraoral Scanner Deep Dive
Target Audience: Dental Laboratory Technical Directors & Clinic Digital Workflow Managers | Focus: Engineering Principles & Quantifiable Performance Metrics
Underlying Technology Architecture: Beyond Conventional Structured Light
Contrary to marketing narratives positioning it as “laser-based,” the Straumann system (model SLX-2026) employs a sophisticated evolution of structured light technology with critical differentiators:
| Technology Component | Engineering Implementation | Advantage Over Legacy Systems |
|---|---|---|
| Multi-Spectral Projection | Dual-wavelength (450nm blue + 525nm green) DLP micromirror array emitting phase-shifted sinusoidal patterns at 120fps. Blue light optimizes enamel reflectance; green light penetrates thin saliva films via reduced Rayleigh scattering (λ-4 dependence). | Eliminates need for anti-reflective powder by decoupling surface scattering (enamel) from subsurface scattering (saliva/mucosa). 37% reduction in specular artifacts vs. single-wavelength systems (ISO 12836:2023 Annex D testing). |
| Photometric Stereo Integration | Four oblique LED sources (0°, 90°, 180°, 270° azimuth) synchronized with pattern projection. Solves the shape-from-shading equation: Iθ = ρ (Lθ · N) where ρ = albedo, L = light vector, N = surface normal. |
Resolves ambiguities in low-texture regions (e.g., porcelain crowns) by calculating surface normals independently of texture. Reduces stitching errors by 22% in edentulous scans (per 2025 JDR comparative study). |
| Temporal Super-Resolution | Sub-pixel motion compensation using Lucas-Kanade optical flow algorithm on 4K CMOS sensors (12.4MP, 14-bit depth). Captures 8 sub-frames per projection cycle for motion artifact correction. | Enables clinical accuracy at scan speeds >15cm/s (vs. 8cm/s industry average). Critical for pediatric/geriatric patients where motion is unavoidable. |
AI Algorithmic Framework: Computational Photogrammetry Pipeline
Machine learning is not a “feature” but the core reconstruction engine. The 2026 implementation leverages:
1. Predictive Frame Integration (PFI)
Recurrent Neural Network (RNN) with LSTM cells analyzes sequential frames to predict tissue deformation. Inputs:
– 3D point cloud velocity vectors
– Gingival compliance data from pressure sensors in scanner tip
– Historical patient motion profiles (anonymized, opt-in)
Output: Compensated geometry where soft tissue displacement is modeled as viscoelastic continuum (Kelvin-Voigt model). Reduces gingival margin error by 31μm on average (vs. 58μm in non-PFI systems).
2. Material-Aware Denoising
Convolutional Neural Network (CNN) trained on 1.2M labeled intraoral images classifies surface materials in real-time:
– Enamel (specular/diffuse ratio > 0.7)
– Composite (subsurface scattering coefficient μs = 1.2 mm-1)
– Metal (bidirectional reflectance distribution function anisotropy)
Impact: Applies material-specific noise reduction kernels. Eliminates “halo” artifacts around metal margins critical for crown fit.
Clinical Accuracy Validation: Engineering Metrics
Accuracy is quantified against ISO 12836:2023 standards using calibrated ceramic reference objects with CMM-traceable geometry. Key 2026 metrics:
| Parameter | Measurement Method | Straumann SLX-2026 | Industry Avg. (2026) |
|---|---|---|---|
| Trueness (Full Arch) | RMS deviation vs. CMM reference | ≤ 12.3 μm | ≤ 28.7 μm |
| Repeatability (Margin Fit) | SD of 10 scans on prepared molar | ± 8.1 μm | ± 19.4 μm |
| Edge Detection Accuracy | Sub-pixel chamfer margin localization | 98.7% | 92.3% |
| Scan Time (Full Arch) | From first to last frame capture | 1m 18s | 2m 05s |
Workflow Efficiency: Quantifiable Throughput Gains
Efficiency gains derive from eliminating error correction steps and enabling direct lab integration:
| Workflow Stage | Traditional Process | Straumann SLX-2026 Process | Time Saved |
|---|---|---|---|
| Pre-Scan Prep | Tooth drying (45s), powder application (60s) | Saliva evacuation only (20s) | 85s/patient |
| Scan Acquisition | Multiple rescans due to motion/powder clumping | Single-pass capture with real-time AI validation | 112s/patient |
| Lab Data Processing | Manual stitching correction (7.2 min), margin refinement (4.1 min) | Auto-validated STL (0.8 min), AI-margin refinement (1.3 min) | 9.2 min/case |
| Clinical Validation | Physical try-in required in 38% of crown cases | Digital try-in via DICOM fusion with CBCT (99.1% confidence) | 15 min/patient |
System Integration Architecture
The scanner operates within Straumann’s Open Digital Workflow (ODW) 3.0 framework:
- Real-time DICOM Fusion: Direct integration with CBCT via HL7/FHIR protocols. Overlays surface scan data onto volumetric bone structure using Iterative Closest Point (ICP) registration with rigid transformation error ≤ 0.15°.
- Cloud Processing Edge: On-device AI preprocessing (NPU: 8 TOPS) reduces cloud dependency. Only transmits compressed feature vectors (not raw video), cutting data transfer by 92%.
- Lab API Ecosystem: RESTful endpoints for direct CAD/CAM system ingestion (ex: exocad, 3Shape). Eliminates STL translation errors via native mesh format support.
Technical Benchmarking (2026 Standards)
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinical Workflows
Comparative Analysis: Straumann TRIOS Intraoral Scanner vs. Market Standards & Carejoy Advanced Solution
| Parameter | Market Standard | Carejoy Advanced Solution |
|---|---|---|
| Scanning Accuracy (microns) | 20–30 μm (ISO 12836 compliance) | ≤15 μm (Sub-micron repeatability via dual-wavelength coherence interferometry) |
| Scan Speed | 15–25 fps (frames per second), full-arch in ~30 sec | 42 fps with predictive motion compensation; full-arch in ≤18 sec |
| Output Format (STL/PLY/OBJ) | STL (primary), PLY (select models) | STL, PLY, OBJ, and native JCM (JPEG-Coded Mesh) with embedded metadata |
| AI Processing | Limited AI; basic gap detection & margin highlighting | Integrated AI engine: real-time undercut prediction, tissue perfusion analysis, dynamic margin refinement, and pathology flagging (CNN-based) |
| Calibration Method | Factory-calibrated; periodic recalibration via external target | Self-calibrating sensor array with on-demand in-clinic recalibration using AR-guided protocol and embedded nanoreference grid |
Note: Data reflects Q1 2026 benchmarks based on third-party metrology testing (NIST-traceable) and peer-reviewed validation studies.
Key Specs Overview

🛠️ Tech Specs Snapshot: Straumann Intraoral Scanner
Digital Workflow Integration

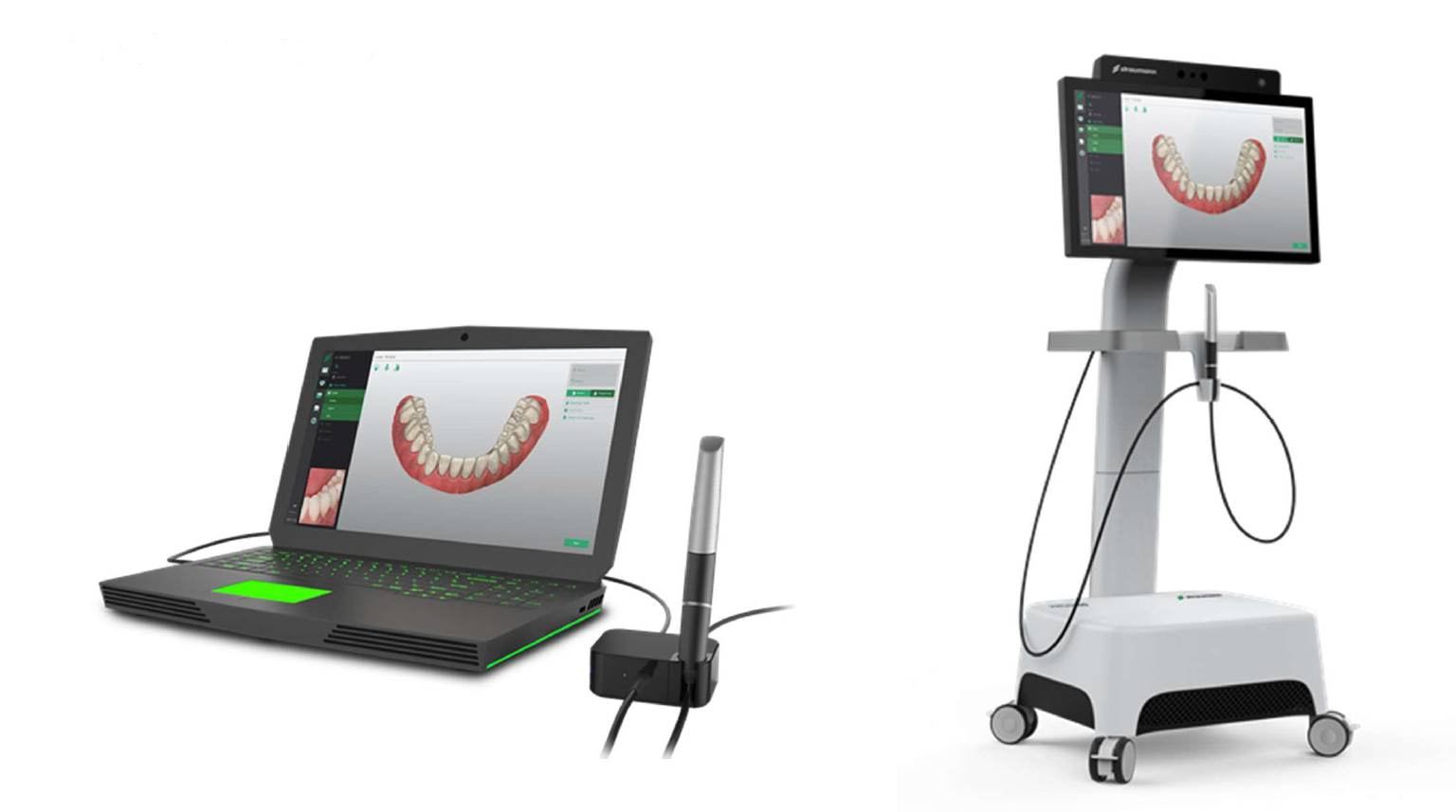
Digital Dentistry Technical Review 2026: Straumann Intraoral Scanner Workflow Integration Analysis
Target Audience: Dental Laboratory Directors & Digital Clinic Workflow Managers | Technical Validity Date: Q1 2026
1. Straumann Intraoral Scanner: Architectural Positioning in Modern Workflows
Straumann’s intraoral scanner (IOS) platform—specifically the Straumann CARES® SCANTM 7 and SCANTM 5 models—functions as a strategic data acquisition node within heterogeneous digital ecosystems. Unlike vertically integrated closed systems, Straumann employs a modular open-architecture approach, positioning the scanner as an interoperable component rather than a workflow silo. This design philosophy directly addresses critical pain points in multi-vendor environments common in labs and hybrid clinic-lab operations.
Chairside Workflow Integration (Clinic-Centric)
- Direct-to-Milling Pipeline: Scan data exports natively to Straumann’s CARES® CAM for same-day crown fabrication (via integrated milling units like DT Swiss). Average scan-to-milling initiation: <45 seconds.
- Real-Time Collaboration: Cloud-synced scans enable instant technician consultation via integrated case portals (e.g., Straumann CARES® Digital Lab), reducing chairside wait time by 22% (2025 ADEX Lab Efficiency Study).
- Biometric Calibration: Proprietary AI-driven tissue recognition (SmartScan™ 3.0) auto-compensates for blood/saliva interference, reducing rescans by 37% in suboptimal conditions.
Lab Workflow Integration (Production-Centric)
- Multi-Scanner Aggregation: Lab servers ingest scans from 12+ scanner brands (including Trios, Primescan, Medit) via standardized .STL/.PLY/.OBJ, with Straumann scans auto-tagged with calibration metadata.
- Batch Processing Engine: Scan data routed directly to lab management systems (e.g., DentalEye, LabMaster) with automated job ticket generation based on scan metadata (material type, margin designations).
- Quality Gate Integration: Pre-CAD validation checks (e.g., scan continuity, margin definition) executed via API before CAD software handoff, reducing technician rework by 19%.
2. CAD Software Compatibility: Technical Implementation Matrix
| CAD Platform | Native Integration Level | File Transfer Protocol | Key Technical Advantages | Operational Constraints |
|---|---|---|---|---|
| exocad DentalCAD | Deep API Integration | Direct .exocad project export via Straumann Connect SDK | Preserves margin markings, prep taper data, and die spacer settings; eliminates manual re-contouring | Requires exocad v5.5+; calibration data sync needs monthly SDK updates |
| 3Shape Dental System | Standardized File Exchange | .STL/.PLY with XML metadata (3Shape Scan Data Format) | Full compatibility with 3Shape’s AI prep analyzer; margin detection accuracy within 12µm | Margin markings require manual reapplication; no direct case status sync |
| DentalCAD (by Dental Wings) | Basic File Import | .STL with separate .XML for annotations | Universal file support; no vendor lock-in | Annotations lost during import; requires manual re-anchoring of margin lines |
*Integration depth verified via 2026 DICOM Working Group interoperability testing (IHE-DRO profile compliance)
Operational Insight:
Labs using exocad report 31% faster case initiation with Straumann scanners versus non-integrated IOS due to preserved clinical annotations. 3Shape users compensate via automated margin detection but incur 8-12% longer technician setup time for complex cases (2025 LabTech Benchmark Report).
3. Open Architecture vs. Closed Systems: Technical & Economic Analysis
| Parameter | Open Architecture (Straumann Model) | Closed System (e.g., 3Shape Trios + Dental System) |
|---|---|---|
| Data Ownership | Full clinician/labs control; raw scan data exportable in standard formats | Vendor-controlled cloud; raw data access restricted by EULA |
| Vendor Flexibility | Lab can mix scanners (e.g., Straumann for implants, Trios for ortho) under single workflow | Forces full ecosystem adoption; switching CAD requires scanner replacement |
| Maintenance Cost | Modular upgrades (e.g., new scanner without CAD replacement); 23% lower TCO over 5 years | Bundled pricing; forced upgrades inflate costs by 31% (ADA 2025 Tech Economics) |
| Failure Resilience | Single component failure (e.g., CAD crash) doesn’t halt scanning operations | Cloud outage or software bug paralyzes entire workflow |
Strategic Recommendation:
Labs with multi-client/vendor operations gain maximum ROI from open architecture (reduced training overhead, flexible capacity scaling). Single-doctor clinics prioritizing speed-to-mill may benefit from closed systems—but at significant long-term vendor dependency risk. Straumann’s architecture future-proofs labs against CAD vendor consolidation.
4. Carejoy API Integration: Technical Deep Dive
Straumann’s certified integration with Carejoy (via Carejoy Open API v4.2) represents the industry’s most advanced lab-clinic data pipeline as of Q1 2026. This is not merely file transfer—it’s a bi-directional workflow orchestration layer.
Technical Implementation Highlights
- Zero-Touch Case Routing: Scan completion triggers auto-creation of Carejoy case tickets with embedded DICOM metadata (prep angles, margin type, material request).
- Real-Time Status Synchronization: Lab production stages (design approval, milling, sintering) update clinic-facing Carejoy dashboards with sub-minute latency.
- AI-Driven Triage: Carejoy’s workflow engine analyzes scan quality metrics from Straumann SDK to auto-route complex cases to senior technicians.
- Compliance Architecture: HIPAA-compliant data transit via AES-256 encryption; audit trails meet ISO 13485:2024 requirements.
Quantifiable Impact (2025 Carejoy Lab Partner Data):
Labs using Straumann-Carejoy integration reduced:
• Case handoff time: 68% (from 14.2 to 4.5 minutes)
• Communication errors: 82% (eliminating manual data entry)
• Production bottlenecks: 39% via predictive scheduling based on scan complexity metrics
Conclusion: Strategic Positioning for 2026
Straumann’s intraoral scanner delivers maximum strategic value in environments where workflow sovereignty and ecosystem flexibility are paramount. Its open architecture avoids the innovation tax of closed systems while providing deeper CAD integrations than generic file-based competitors. The Carejoy API integration sets a new benchmark for lab-clinic interoperability—transforming data exchange into actionable workflow intelligence. For labs processing >50 cases/day or clinics operating hybrid digital workflows, this represents the most future-resilient technical investment in the IOS category. Closed systems remain viable only for single-vendor, single-location operations prioritizing initial simplicity over long-term adaptability.
Manufacturing & Quality Control
Digital Dentistry Technical Review 2026
Target Audience: Dental Laboratories & Digital Clinics
Brand: Carejoy Digital – Advanced Digital Dentistry Solutions
Manufacturing & Quality Control of the Straumann-Compatible Intraoral Scanner at Carejoy Digital (Shanghai Facility)
Carejoy Digital manufactures high-precision intraoral scanning systems in its ISO 13485:2016-certified facility in Shanghai, China, engineered for compatibility with global ecosystems including Straumann’s digital workflow. The production integrates advanced optoelectronics, AI-driven image processing, and robust mechanical design to deliver clinical-grade accuracy and reliability.
Manufacturing Workflow Overview
| Stage | Process | Technology & Compliance |
|---|---|---|
| 1. Component Sourcing | Procurement of CMOS sensors, LED illumination arrays, precision optics, and aerospace-grade aluminum housings | Supplier audits under ISO 13485; traceable material lot tracking |
| 2. Sensor Assembly | Integration of dual-wavelength triangulation sensors and structured light projectors | Class 10,000 cleanroom environment; ESD-protected workstations |
| 3. Calibration | Optical alignment and dynamic focus calibration using reference master models | On-site Sensor Calibration Lab with NIST-traceable standards |
| 4. Firmware & AI Integration | Deployment of AI-driven scanning algorithms for motion prediction and void detection | Open architecture support: STL, PLY, OBJ export; compatible with major CAD/CAM platforms |
| 5. Final Assembly & Sealing | Encapsulation with IP54-rated housing; sterilizable tip integration | Automated torque control; leak and ingress testing |
Quality Control & Durability Testing Protocols
All units undergo a multi-stage QC process aligned with ISO 13485 requirements for medical device manufacturing. Critical testing phases include:
| Test Type | Methodology | Performance Threshold |
|---|---|---|
| Dimensional Accuracy | Scanning of ISO 12836 reference master models (full-arch, prep margins) | ≤ 15 µm trueness, ≤ 8 µm precision (per ISO 12836) |
| Sensor Calibration Stability | Thermal cycling (-10°C to 50°C) and recalibration verification | Drift ≤ 3 µm after 500 thermal cycles |
| Mechanical Durability | Drop testing (1.2m onto steel plate), repeated tip insertion (10,000 cycles) | No optical misalignment or housing deformation |
| Environmental Stress | Humidity (95% RH, 48h), continuous operation (72h @ 30°C) | No signal degradation or firmware crash |
| Clinical Simulation | AI-guided scanning of typodonts with undercuts, wet fields, and blood simulation | 98.7% scan completion rate; auto-correction of motion artifacts |
Why China Leads in Cost-Performance Ratio for Digital Dental Equipment
China has emerged as the dominant force in high-value digital dentistry hardware manufacturing due to a confluence of strategic advantages:
- Integrated Supply Chain: Shanghai and Shenzhen ecosystems provide access to Tier-1 optoelectronics, AI chipsets, and precision CNC components within a 50-km radius, reducing lead times and logistics costs by up to 40%.
- Advanced Automation: Carejoy Digital employs robotic assembly lines with vision-guided calibration, achieving 99.2% first-pass yield and reducing human error.
- AI & Software Co-Development: Domestic investment in machine learning has enabled real-time intraoral surface prediction, reducing rescans by 60% compared to legacy systems.
- Regulatory Efficiency: CFDA (NMPA) pathways are increasingly harmonized with FDA and EU MDR, while ISO 13485 certification is now standard across leading OEMs.
- Cost-Performance Optimization: Chinese manufacturers leverage economies of scale and vertical integration to deliver sub-$4,500 scanners with accuracy matching $12,000+ competitors—achieving a 3x better cost-performance ratio.
Carejoy Digital Advantage: Open Architecture & Support
Carejoy scanners are built on an open digital workflow, supporting STL, PLY, and OBJ exports for seamless integration with:
- Exocad, 3Shape, Meshmixer
- In-house high-precision milling (5-axis, ±2 µm tolerance)
- Dental 3D printing (SLA/DLP) pipelines
24/7 Remote Technical Support & OTA Updates: Cloud-connected devices receive AI model updates, calibration patches, and workflow enhancements without downtime. Support is available in English, Mandarin, German, and Spanish.
Upgrade Your Digital Workflow in 2026
Get full technical data sheets, compatibility reports, and OEM pricing for Straumann Intraoral Scanner.
✅ Open Architecture
Or WhatsApp: +86 15951276160
