Introduction: Navigating the Global Market for negatives of dental implants
Navigating the global market for dental implants requires a nuanced understanding of not only the benefits but also the potential negatives associated with these products. For B2B buyers, particularly those from regions such as Africa, South America, the Middle East, and Europe, recognizing the challenges and limitations of dental implants is essential for making informed purchasing decisions. This guide delves into the critical aspects that buyers must consider, including the various types of implants, the materials used, manufacturing and quality control standards, and the landscape of suppliers.
Understanding the negatives of dental implants can significantly impact sourcing strategies. Issues such as compatibility, potential for complications, and varying success rates across different demographics must be weighed against the expected benefits. By exploring these factors, buyers can better assess the risks involved and select products that align with their market needs and patient expectations.
This comprehensive guide also provides valuable insights into cost considerations and market trends, helping international buyers navigate pricing structures and supply chain dynamics effectively. With an emphasis on frequently asked questions, we aim to clarify common concerns and equip buyers with the knowledge needed to engage confidently with suppliers.
In an increasingly competitive landscape, having a thorough grasp of the negatives associated with dental implants not only enhances decision-making but also empowers businesses to foster trust and deliver quality care to their clients.
Understanding negatives of dental implants Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Surgical Complications | Risk of infection, nerve damage, or sinus issues | Dental clinics, surgical centers | Pros: High success rate. Cons: Potential for serious complications. |
| Material Reactions | Allergic reactions to titanium or other implant materials | Dental suppliers, implant manufacturers | Pros: Durable materials. Cons: Risk of material rejection. |
| Bone Integration Failure | Inadequate osseointegration due to poor bone quality | Hospitals, dental practices | Pros: Long-lasting solution. Cons: May require additional procedures. |
| Cost Factors | High initial investment and ongoing maintenance costs | Insurance companies, dental financing services | Pros: Long-term investment in oral health. Cons: High upfront costs. |
| Aesthetic Concerns | Visible implant components or gum recession | Cosmetic dentistry, orthodontic practices | Pros: Improved appearance. Cons: Potential for aesthetic issues. |
Surgical Complications
Surgical complications can arise during or after the dental implant procedure, including infections, nerve damage, or sinus issues. These complications may lead to significant additional costs and prolonged recovery times. B2B buyers should consider partnering with experienced surgical centers that prioritize patient safety and have a track record of minimizing such risks. Understanding the potential complications is crucial for dental practices aiming to provide high-quality care while managing patient expectations.
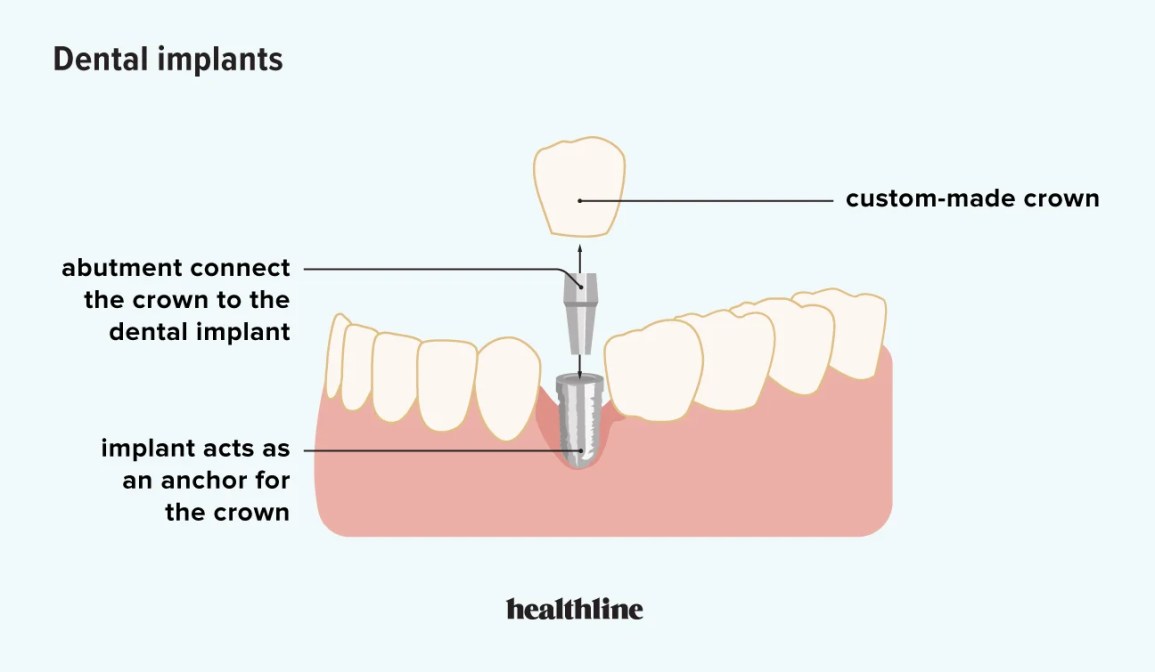
Illustrative Image (Source: Google Search)
Material Reactions
Material reactions refer to allergic responses that patients may have to titanium or other implant materials. While titanium is widely regarded for its biocompatibility, certain individuals may still experience adverse reactions. B2B buyers need to evaluate the materials used in implants and consider offering alternatives, such as zirconia implants, which may be better tolerated by sensitive patients. This consideration can enhance patient satisfaction and expand market offerings.
Bone Integration Failure
Bone integration failure occurs when the dental implant does not properly fuse with the jawbone, often due to insufficient bone density or quality. This issue can necessitate additional surgical procedures, such as bone grafting, which can increase costs and extend treatment timelines. For B2B buyers, it is essential to assess the bone quality of potential patients and invest in technologies that promote successful osseointegration. This proactive approach can reduce the likelihood of failure and improve overall patient outcomes.
Cost Factors
The financial implications of dental implants can be significant, with high initial investments and ongoing maintenance costs. B2B buyers, such as dental practices and suppliers, should be prepared to navigate these financial aspects, including patient financing options and insurance partnerships. Offering transparent pricing and flexible payment plans can attract more clients and facilitate better patient access to implant solutions, ultimately enhancing business growth.
Aesthetic Concerns
Aesthetic concerns arise from visible implant components or gum recession, which can impact a patient’s overall appearance. Dental practices must prioritize aesthetic outcomes alongside functional benefits when recommending implants. B2B buyers should consider investing in high-quality materials and techniques that minimize visibility and promote natural-looking results. By addressing aesthetic concerns, dental providers can enhance patient satisfaction and foster long-term loyalty, crucial for sustaining a competitive edge in the market.
Related Video: All on 4 Dental Implants Explained
Key Industrial Applications of negatives of dental implants
| Industry/Sector | Specific Application of negatives of dental implants | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Dental Manufacturing | Creating precise prosthetic molds | Enhanced accuracy in fit, reducing remakes and costs | Quality of materials, precision of the negatives, supplier certifications |
| Dental Clinics | Patient-specific treatment planning | Improved patient outcomes and satisfaction | Availability of customization options, turnaround time, and support services |
| Research and Development | Testing and prototyping new dental technologies | Accelerated innovation and market readiness | Access to advanced materials, collaboration capabilities, and regulatory compliance |
| Education and Training | Simulated training for dental professionals | Better skill acquisition and confidence in procedures | Quality of simulation materials, realism of training aids, and cost-effectiveness |
| Dental Laboratories | Quality control in implant production | Reduced defects and improved product reliability | Supplier reliability, material consistency, and technological capabilities |
Detailed Applications
Dental Manufacturing
In the dental manufacturing sector, negatives of dental implants are crucial for creating precise prosthetic molds. These negatives provide the exact shape and dimensions needed for dental prosthetics, ensuring a perfect fit for patients. This precision reduces the likelihood of remakes, which can be costly and time-consuming. For international buyers, it’s essential to consider the quality of materials used in negatives, as well as the precision of the negatives produced. Supplier certifications and quality assurance processes are also critical to ensure that the products meet industry standards.
Dental Clinics
Dental clinics utilize negatives of dental implants for patient-specific treatment planning. By using these negatives, dental professionals can create customized solutions that cater to the unique anatomical features of each patient. This customization leads to improved patient outcomes and higher satisfaction rates. Buyers in this sector should prioritize suppliers that offer a range of customization options, quick turnaround times, and robust support services, particularly in regions where dental technology may be evolving rapidly.
Research and Development
In the realm of research and development, negatives of dental implants play a pivotal role in testing and prototyping new dental technologies. They allow for the simulation of various implant designs and materials, facilitating rapid innovation. For B2B buyers, especially those in regions with burgeoning dental tech markets, access to advanced materials and collaboration capabilities with suppliers can significantly enhance their product development cycles. Additionally, understanding regulatory compliance is vital to ensure that any new product meets necessary industry standards.
Education and Training
Negatives of dental implants are also employed in the education and training of dental professionals. They provide realistic simulations for dental procedures, allowing trainees to practice and hone their skills without the risk associated with real patients. This application not only boosts the confidence of new professionals but also improves overall treatment quality. Buyers in this sector should evaluate the quality of simulation materials, the realism of training aids, and the cost-effectiveness of these educational tools to ensure they provide the best learning experiences.
Dental Laboratories
In dental laboratories, negatives of dental implants are used for quality control during implant production. They help identify defects early in the manufacturing process, leading to improved product reliability and reduced waste. For international buyers, the reliability of suppliers and consistency of materials are paramount. Understanding the technological capabilities of suppliers can also influence the efficiency and quality of production processes, making these factors critical in the sourcing decision.
Related Video: 3 Types of Dental Implants and Surface treatments explained!
Strategic Material Selection Guide for negatives of dental implants
When selecting materials for dental implants, particularly in the context of their negatives, it is essential to understand the properties, advantages, and limitations of commonly used materials. This analysis focuses on titanium, zirconia, cobalt-chromium alloys, and polymer-based materials, providing actionable insights for international B2B buyers.
Titanium
Key Properties: Titanium is renowned for its excellent strength-to-weight ratio, corrosion resistance, and biocompatibility. It can withstand significant pressure and has a high melting point, making it suitable for various environments.
Pros & Cons: Titanium implants are durable and have a long track record in dental applications, contributing to their widespread acceptance. However, they can be expensive due to manufacturing complexity and the need for precise machining. Additionally, titanium may cause allergic reactions in some patients, which is a significant consideration for healthcare providers.
Impact on Application: Titanium is compatible with various oral environments, including the presence of saline and other bodily fluids. However, its use may be limited in patients with specific allergies.
Considerations for International Buyers: Buyers should ensure compliance with international standards such as ISO 13485 for medical devices and ASTM F136 for titanium alloys. In regions like Europe and the Middle East, regulatory approvals may require extensive documentation.
Zirconia
Key Properties: Zirconia is a ceramic material known for its high strength and toughness, along with excellent aesthetic properties. It is also highly resistant to wear and corrosion.
Pros & Cons: The aesthetic appeal of zirconia makes it a preferred choice for visible dental implants. However, it is generally more brittle than titanium, which can lead to fracture under stress. The manufacturing process can also be complex and costly, impacting overall pricing.
Impact on Application: Zirconia is particularly suitable for patients who prioritize aesthetics, as it can be easily colored to match natural teeth. However, its brittleness may limit its use in load-bearing applications.
Considerations for International Buyers: Compliance with ISO standards, particularly ISO 6872 for dental ceramics, is crucial. Additionally, buyers in regions like South America and Africa should consider local acceptance of zirconia implants, which may vary based on market maturity.
Cobalt-Chromium Alloys
Key Properties: Cobalt-chromium alloys are characterized by their high strength, corrosion resistance, and wear resistance. They can withstand high temperatures, making them suitable for various dental applications.
Pros & Cons: These alloys are highly durable and resistant to deformation, making them suitable for long-term use. However, they can be more expensive than titanium and zirconia and may cause allergic reactions in sensitive patients.
Impact on Application: Cobalt-chromium is often used in frameworks for dental prosthetics due to its strength and compatibility with different media. However, its use in direct implants may be limited due to biocompatibility concerns.
Considerations for International Buyers: Buyers should ensure that cobalt-chromium products comply with ASTM F75 standards. In regions like the Middle East, where regulatory scrutiny is increasing, thorough documentation and testing may be required.
Polymer-Based Materials
Key Properties: Polymer-based materials, such as polyether ether ketone (PEEK), offer flexibility, lightweight properties, and resistance to chemical degradation. They are also biocompatible.
Pros & Cons: These materials are often less expensive and easier to manufacture than metals or ceramics. However, they may not provide the same level of durability and strength, which can be a significant drawback in load-bearing applications.
Impact on Application: Polymers are suitable for temporary implants or as a component in hybrid solutions. Their compatibility with various bodily fluids makes them versatile, but their mechanical properties may limit their use in permanent applications.
Considerations for International Buyers: Compliance with ISO 10993 for biocompatibility is essential. Buyers should also be aware of regional preferences, as polymer use in dental applications may be less established in certain markets compared to metals and ceramics.
Summary Table
| Material | Typical Use Case for negatives of dental implants | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Titanium | Permanent dental implants | Excellent strength and biocompatibility | High cost and potential allergic reactions | High |
| Zirconia | Aesthetic dental implants | Superior aesthetics | Brittle and costly | Med |
| Cobalt-Chromium Alloys | Frameworks for dental prosthetics | High durability and wear resistance | Higher cost and potential allergies | High |
| Polymer-Based Materials | Temporary implants or hybrid solutions | Cost-effective and lightweight | Lower durability in load-bearing scenarios | Low |
In-depth Look: Manufacturing Processes and Quality Assurance for negatives of dental implants
Manufacturing Processes for Negatives of Dental Implants
The manufacturing of negatives for dental implants involves a series of meticulously controlled processes designed to ensure high precision and quality. Understanding these processes can provide B2B buyers with insights into product reliability and supplier capability.
Main Stages of Manufacturing
-
Material Preparation
– Selection of Materials: The first step involves selecting biocompatible materials, such as high-grade polymers or silicone, which are crucial for creating accurate impressions. The choice of material affects the durability and accuracy of the negatives.
– Material Testing: Prior to production, materials undergo testing for physical properties, such as tensile strength and elasticity, ensuring they meet industry standards. -
Forming
– Molding Techniques: The forming process typically utilizes injection molding or vacuum forming. Injection molding allows for precise replication of the dental anatomy, while vacuum forming is often used for less complex shapes.
– Temperature Control: Maintaining optimal temperature during the molding process is essential to avoid defects and ensure consistent quality. -
Assembly
– Integration of Components: In cases where negatives consist of multiple parts, assembly must be performed with precision to ensure proper fit and function.
– Use of Adhesives: When components are bonded, the selection of appropriate adhesives is critical to maintain the integrity of the negatives. -
Finishing
– Surface Treatment: Post-manufacturing, surfaces may undergo treatments such as polishing or coating to enhance accuracy and durability.
– Final Inspection: This stage includes visual and dimensional inspections to confirm that the negatives meet specified tolerances.
Quality Assurance in Dental Implant Negatives
Quality assurance (QA) is paramount in the production of dental implant negatives. B2B buyers should be familiar with various standards and practices that ensure product quality and safety.
International Standards
- ISO 9001: This quality management standard ensures that manufacturers maintain consistent quality in their processes and products. Buyers should verify that their suppliers are certified to this standard.
- CE Marking: For products sold in Europe, CE marking indicates compliance with health, safety, and environmental protection standards. It is essential for ensuring that the negatives meet European regulations.
- API Standards: In some regions, adherence to American Petroleum Institute (API) standards may also be relevant, particularly for materials used in dental applications.
Key Quality Control Checkpoints
-
Incoming Quality Control (IQC)
– This involves inspecting raw materials upon arrival to ensure they meet predefined specifications. Buyers should request IQC reports from suppliers. -
In-Process Quality Control (IPQC)
– Ongoing monitoring during the manufacturing process helps catch defects early. This may involve statistical process control (SPC) methods to analyze production data. -
Final Quality Control (FQC)
– A comprehensive examination of the finished products to ensure they meet all quality standards. This includes dimensional checks and surface inspections.
Common Testing Methods
- Dimensional Analysis: Utilizing calipers and 3D scanning technology to verify that the negatives match the required specifications.
- Biocompatibility Testing: Conducting tests to ensure that materials used in the negatives do not provoke adverse reactions when in contact with biological tissues.
- Mechanical Testing: Evaluating the durability and performance of the negatives under simulated conditions to ensure they can withstand clinical use.
Verifying Supplier Quality Control
B2B buyers should take proactive steps to verify the quality control processes of their suppliers:
- Supplier Audits: Conducting regular audits of suppliers can provide firsthand insights into their manufacturing processes and quality assurance practices.
- Requesting Quality Reports: Buyers should ask for detailed quality reports that outline the results of IQC, IPQC, and FQC processes.
- Third-Party Inspections: Engaging independent third-party inspectors can provide an objective assessment of the supplier’s manufacturing and quality assurance capabilities.
Quality Control and Certification Nuances for International Buyers
When sourcing from suppliers in different regions, B2B buyers must consider various nuances:
- Regulatory Compliance: Buyers from Africa, South America, the Middle East, and Europe must be aware of their local regulations regarding dental products. This includes understanding the certification requirements specific to each region.
- Cultural and Operational Differences: Each region may have unique practices and attitudes toward quality assurance. Buyers should be prepared to adapt their verification processes accordingly.
- Documentation and Traceability: Ensuring that suppliers provide comprehensive documentation for traceability is crucial. This includes records of all manufacturing processes, materials used, and quality checks performed.
Conclusion
Understanding the manufacturing processes and quality assurance measures for dental implant negatives is essential for international B2B buyers. By focusing on material selection, manufacturing techniques, and rigorous quality control practices, buyers can enhance their supply chain’s reliability and ensure they are sourcing high-quality products. Engaging with suppliers through audits and requiring detailed reports will further solidify confidence in the products being procured, ultimately leading to better outcomes in dental implant procedures.
Related Video: Inspection and Quality control in Manufacturing
Comprehensive Cost and Pricing Analysis for negatives of dental implants Sourcing
Understanding the cost structure and pricing dynamics of sourcing dental implants is crucial for international B2B buyers. This analysis delves into the various cost components, price influencers, and actionable insights tailored for buyers from Africa, South America, the Middle East, and Europe.
Cost Components
-
Materials: The cost of raw materials—such as titanium, zirconia, and biocompatible polymers—forms the foundation of the overall expense. Prices can fluctuate based on market demand and availability. Buyers should consider sourcing from multiple suppliers to mitigate risks associated with material shortages.
-
Labor: Labor costs are significant in the manufacturing process of dental implants. Skilled labor is essential for precision and quality, especially in regions with high labor standards. Countries with lower labor costs can offer competitive pricing, but this may come at the expense of quality.
-
Manufacturing Overhead: This includes costs related to factory operations, utilities, and equipment maintenance. Efficient manufacturing processes can lower overhead costs. Buyers should inquire about the supplier’s operational efficiencies, as these can directly impact pricing.
-
Tooling: The initial investment in tooling for production can be substantial. Custom tooling for specific implant designs can increase costs. Buyers should evaluate whether off-the-shelf solutions may suffice for their needs to keep costs in check.
-
Quality Control (QC): Rigorous QC processes are necessary to ensure the safety and efficacy of dental implants. While this adds to the overall cost, it is essential for maintaining compliance with international standards. Suppliers with robust QC systems may justify higher prices through better product reliability.
-
Logistics: Shipping and handling costs vary significantly based on distance, mode of transport, and regional regulations. Buyers should consider Incoterms to understand who bears the cost and risks associated with logistics.
-
Margin: Supplier margins can vary based on their market position, brand reputation, and service offerings. Buyers should compare multiple suppliers to gauge the standard margin for similar products.
Price Influencers
-
Volume/MOQ: Bulk purchasing can lead to significant discounts. Establishing a Minimum Order Quantity (MOQ) can provide leverage in negotiations and reduce per-unit costs.
-
Specifications/Customization: Custom implants designed for specific patient needs can incur additional costs. Buyers should assess the necessity of customization against potential budget constraints.
-
Quality/Certifications: Implants that meet international standards (e.g., ISO, CE marking) may command higher prices but are often worth the investment due to reduced liability and improved patient outcomes.
-
Supplier Factors: The supplier’s reputation, production capabilities, and geographical location can influence pricing. Buyers should conduct due diligence to ensure they partner with reputable suppliers.
-
Incoterms: Understanding Incoterms is vital for international transactions. They define responsibilities between buyers and sellers, impacting logistics and total costs.
Buyer Tips
-
Negotiation: Leverage competitive offers from multiple suppliers to negotiate better terms. Highlighting your purchasing power can lead to favorable pricing agreements.
-
Cost-Efficiency: Focus on the Total Cost of Ownership (TCO) rather than just the initial purchase price. This includes potential costs related to warranties, maintenance, and product lifespan.
-
Pricing Nuances: Be aware of regional pricing variations. For example, import tariffs in Africa or South America can significantly impact costs. Understanding local market dynamics can aid in effective budget planning.
-
Research and Development: Engage with suppliers that invest in R&D for innovative solutions. Advanced technologies can lead to cost savings in the long term through enhanced product performance and reduced complications.
Disclaimer
Prices are indicative and can vary based on multiple factors including market conditions, supplier negotiations, and specific buyer requirements. Always conduct thorough research and obtain multiple quotes to ensure competitive pricing.
Spotlight on Potential negatives of dental implants Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘negatives of dental implants’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for negatives of dental implants
Key Technical Properties of Dental Implant Negatives
Understanding the technical specifications related to the negatives of dental implants is crucial for B2B buyers. These properties not only influence the quality and effectiveness of the implants but also affect procurement decisions and supplier negotiations.
-
Material Grade: The material used in dental implant negatives typically includes high-grade polymers or silicone. Material grade determines the strength, durability, and biocompatibility of the implant. For buyers, selecting the right material grade is essential to ensure that the product meets regulatory standards and patient safety requirements.
-
Dimensional Tolerance: This refers to the permissible limits of variation in the dimensions of the negatives. High precision is critical, as even minor deviations can affect the fit and functionality of the implant. Buyers should prioritize suppliers who can provide detailed tolerances to ensure compatibility with existing dental systems.
-
Surface Finish: The texture and finish of the negatives can significantly impact the adhesion properties and ease of use in dental procedures. A smooth finish may facilitate easier handling and application, while a textured surface might enhance bonding capabilities. B2B buyers should consider how the surface finish aligns with their clinical needs.
-
Thermal Stability: Dental implant negatives must withstand various sterilization processes without degrading. Buyers should inquire about the thermal stability of the materials used to ensure they can endure repeated sterilization cycles without compromising integrity.
-
Color Stability: Over time, exposure to light and other environmental factors can affect the color of dental implant negatives. Color stability is particularly important for aesthetic applications. Buyers should seek materials that maintain their color integrity over time to ensure consistent results.
Common Trade Terminology
Familiarity with industry jargon is vital for effective communication and negotiation in the dental implant market. Here are several key terms that B2B buyers should understand:
-
OEM (Original Equipment Manufacturer): This term refers to companies that produce parts or equipment that may be marketed by another manufacturer. Understanding OEM relationships can help buyers identify reliable sources for dental implant negatives that meet their specifications.
-
MOQ (Minimum Order Quantity): MOQ is the smallest quantity of a product that a supplier is willing to sell. B2B buyers should be aware of the MOQ to optimize inventory levels and ensure they do not overcommit financially, especially in emerging markets.
-
RFQ (Request for Quotation): An RFQ is a formal document issued by a buyer to solicit price quotes from suppliers. It is essential for establishing a competitive pricing environment. Buyers should prepare detailed RFQs to receive accurate and comparable quotes.
-
Incoterms (International Commercial Terms): These are internationally recognized standard trade terms that define the responsibilities of buyers and sellers in international transactions. Understanding Incoterms is crucial for managing shipping costs and risks associated with the delivery of dental implant negatives.
-
Lead Time: This refers to the time between placing an order and receiving the product. Knowing lead times can help buyers manage their supply chains more effectively, ensuring that they have the necessary materials when needed.
-
Certifications: Various certifications may apply to dental implant negatives, such as ISO or CE marks. These certifications indicate compliance with international quality and safety standards. Buyers should verify certifications to ensure that the products meet regulatory requirements in their respective markets.
By grasping these technical properties and trade terms, B2B buyers can make informed decisions that align with their operational needs and market demands, ultimately leading to more successful procurement strategies in the dental implant sector.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the negatives of dental implants Sector
Market Overview & Key Trends
The dental implant sector is currently experiencing significant shifts driven by technological advancements, evolving consumer preferences, and regulatory changes. For international B2B buyers, especially in regions like Africa, South America, the Middle East, and Europe, understanding these dynamics is crucial for effective sourcing strategies. One of the key trends is the increasing demand for minimally invasive procedures, which has led to the development of innovative implant designs and materials that promise better patient outcomes. Additionally, the rise of digital dentistry—such as 3D printing and computer-aided design (CAD)—is transforming how implants are produced and fitted, providing opportunities for cost reduction and improved precision.
Illustrative Image (Source: Google Search)
Another critical market dynamic is the growing emphasis on product safety and quality. Regulatory bodies are tightening their scrutiny over the dental implant supply chain, making it essential for buyers to ensure compliance with international standards. In this landscape, establishing relationships with reputable manufacturers and suppliers who demonstrate transparency in their processes is vital. Furthermore, buyers should be aware of the regional disparities in market maturity; for instance, while Europe showcases advanced technologies and higher standards, emerging markets in Africa and South America may still grapple with issues related to product quality and availability.
Sustainability & Ethical Sourcing in B2B
Sustainability has become a cornerstone of decision-making in the dental implant sector. The environmental impact of dental implants—ranging from the extraction of raw materials to waste generated during production—cannot be overlooked. B2B buyers are increasingly prioritizing suppliers who adopt sustainable practices, such as utilizing recycled materials or eco-friendly manufacturing processes.
Moreover, ethical sourcing is paramount in building a responsible supply chain. Buyers should seek partnerships with manufacturers that adhere to ethical labor practices and demonstrate a commitment to reducing their carbon footprint. Certifications such as ISO 14001 (Environmental Management) and ISO 26000 (Social Responsibility) can serve as indicators of a supplier’s commitment to sustainability. By opting for ‘green’ certified products, businesses not only contribute to environmental conservation but also enhance their brand reputation among environmentally-conscious consumers.
Brief Evolution/History
The dental implant industry has evolved significantly since the first modern implants were introduced in the 1960s. Initially, the focus was primarily on functionality, with materials such as titanium dominating the market due to their biocompatibility and strength. Over the decades, however, the industry has seen a shift towards more patient-centric approaches, emphasizing aesthetics, comfort, and long-term sustainability. As technological advancements continue to emerge, the market is also witnessing the introduction of new materials and methods that address previous shortcomings, such as implant failure rates and aesthetic concerns. This historical evolution underscores the need for B2B buyers to stay informed about advancements and shifts in consumer demands to make educated sourcing decisions.
Related Video: Global Trends Tutorial: Chapter 3: IPE
Frequently Asked Questions (FAQs) for B2B Buyers of negatives of dental implants
-
What are the primary concerns regarding the quality of dental implants from international suppliers?
Quality control is critical when sourcing dental implants internationally. B2B buyers should prioritize suppliers with robust quality assurance certifications, such as ISO 13485, which indicates compliance with international standards for medical devices. Conducting on-site audits and requiring samples for testing can further mitigate risks. Additionally, buyers should verify the supplier’s history, including any recalls or quality issues, to assess their reliability. Establishing clear quality benchmarks in contracts can also help in holding suppliers accountable. -
How can I ensure the customization of dental implants meets specific market needs?
Customization is essential for addressing varying patient needs across different markets. B2B buyers should engage in detailed discussions with suppliers about their capabilities for customization, including materials, sizes, and designs. Requesting prototypes and conducting market research to understand local preferences can guide this process. It’s beneficial to establish a collaborative relationship with suppliers to foster innovation and tailor products effectively. Ensure that customization options align with regulatory standards in your target markets. -
What are the typical minimum order quantities (MOQs) for dental implants, and how do they affect pricing?
MOQs for dental implants can vary significantly by supplier and product type. Generally, higher MOQs can lead to lower per-unit costs, which is advantageous for bulk buyers. However, B2B buyers should assess whether the MOQ aligns with their market demand to avoid excess inventory. Negotiate MOQs that provide flexibility, especially if you’re entering a new market. Consider discussing tiered pricing structures where lower MOQs are rewarded with slightly higher prices, allowing for better cash flow management. -
What should I know about lead times when sourcing dental implants internationally?
Lead times can significantly impact inventory management and sales strategies. B2B buyers must communicate clearly with suppliers about expected lead times and any factors that may cause delays, such as manufacturing capacity or logistics challenges. It is advisable to factor in additional time for customs clearance and potential disruptions. Establishing a reliable forecast for your orders can help suppliers plan better, potentially reducing lead times. Always have contingency plans in place for urgent orders. -
How can I ensure compliance with international standards and certifications when sourcing dental implants?
Ensuring compliance with international standards is crucial for market entry and patient safety. B2B buyers should request documentation proving that suppliers adhere to relevant certifications such as CE marking in Europe or FDA approval in the United States. Conducting due diligence, including third-party audits or consulting with regulatory experts, can help verify compliance. It’s also beneficial to stay updated on regulatory changes in your target markets, as non-compliance can lead to costly delays and penalties. -
What logistics considerations should I keep in mind when importing dental implants?
Logistics play a vital role in the successful importation of dental implants. B2B buyers should evaluate shipping options, including air freight for speed or sea freight for cost-effectiveness. Understanding the full supply chain, from the supplier’s location to your distribution center, is essential. Be mindful of customs regulations and import duties that could affect overall costs. Partnering with experienced logistics providers can streamline the process and help navigate any challenges that arise during transportation. -
What steps can I take to resolve disputes with suppliers regarding dental implants?
Disputes can arise from various issues, including quality, delivery, or payment terms. B2B buyers should have clear contracts outlining expectations, responsibilities, and dispute resolution procedures. Establishing a communication protocol for addressing concerns promptly can prevent escalation. If disputes do occur, consider mediation or arbitration as a first step before resorting to legal action, which can be costly and time-consuming. Maintaining a professional relationship with suppliers is key to resolving issues amicably. -
How do I assess the overall reputation of a supplier in the dental implant industry?
Assessing a supplier’s reputation involves thorough research and due diligence. Start by checking online reviews, testimonials, and case studies from other B2B buyers in your industry. Networking at industry trade shows and conferences can provide insights into suppliers’ reliability and product quality. Additionally, consider reaching out to industry associations or regulatory bodies for recommendations. A supplier with a solid track record of customer satisfaction and compliance is generally a safer choice for your business.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for negatives of dental implants
In conclusion, understanding the negatives of dental implants is crucial for international B2B buyers. Key considerations include the potential for complications, varying patient acceptance, and the financial implications associated with sourcing high-quality materials and skilled professionals. These challenges highlight the importance of strategic sourcing—a process that allows businesses to mitigate risks by thoroughly evaluating suppliers, ensuring compliance with international standards, and investing in training for dental professionals.
As buyers from regions such as Africa, South America, the Middle East, and Europe navigate the complex landscape of dental implants, it is essential to prioritize partnerships with suppliers who demonstrate a commitment to quality and innovation. By leveraging strategic sourcing techniques, businesses can enhance their product offerings while addressing the inherent risks associated with dental implants.
Looking ahead, the demand for dental implants will continue to grow, driven by advancements in technology and an increasing focus on oral health. International B2B buyers are encouraged to stay informed about market trends and to actively seek out suppliers who align with their strategic goals. Embrace the opportunity to build resilient supply chains that not only meet current needs but also adapt to future challenges in the dental industry.
