Introduction: Navigating the Global Market for single tooth dental implants
In the rapidly evolving dental landscape, single tooth dental implants have emerged as a cornerstone for restorative dentistry. These implants not only restore functionality and aesthetics but also enhance the quality of life for patients, making them a critical investment for dental practices and suppliers alike. As international B2B buyers seek to navigate this complex market, understanding the nuances of single tooth implants becomes essential.
This comprehensive guide delves into various aspects of single tooth dental implants, including the different types available, materials used, and manufacturing quality control processes. Additionally, it offers insights into sourcing strategies, reputable suppliers, and pricing considerations tailored to the unique needs of buyers from Africa, South America, the Middle East, and Europe.
By addressing frequently asked questions and providing actionable insights, this guide empowers B2B buyers to make informed decisions that align with their operational goals. Whether you are seeking to expand your product offerings or optimize your supply chain, understanding the dynamics of single tooth dental implants will enhance your competitive edge in the global market. This resource is designed to equip you with the knowledge necessary to navigate the complexities of sourcing and ensure successful procurement strategies in a diverse and growing industry.
Understanding single tooth dental implants Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Endosteal Implants | Placed directly into the jawbone; most common type. | General dentistry, oral surgery | Pros: High success rate; suitable for most patients. Cons: Requires sufficient bone density; longer healing time. |
| Subperiosteal Implants | Placed under the gum but above the jawbone; used when bone is insufficient. | Prosthodontics, specialized dental practices | Pros: No need for bone grafting; stable for patients with bone loss. Cons: More invasive; higher cost. |
| Zirconia Implants | Made from zirconia ceramic; aesthetic and biocompatible. | Cosmetic dentistry, high-end dental practices | Pros: Excellent aesthetic appeal; metal-free. Cons: Limited long-term data; can be more expensive. |
| One-Piece Implants | Integrated design combining post and abutment; simpler procedure. | Fast-track dental clinics, urgent care | Pros: Reduced treatment time; lower infection risk. Cons: Not suitable for all cases; less flexibility in adjustments. |
| Mini Implants | Smaller diameter; ideal for narrow spaces and temporary solutions. | Orthodontics, immediate denture stabilization | Pros: Minimally invasive; quicker placement. Cons: Lower stability; not ideal for heavy loads. |
Endosteal Implants
Endosteal implants are the most prevalent type of dental implant, designed to be inserted directly into the jawbone. They are typically made of titanium, providing a robust foundation for the prosthetic tooth. B2B buyers should consider the implant’s success rate, which is generally high, making it suitable for most patients. However, adequate bone density is required for placement, and the healing time can be longer compared to other types.
Subperiosteal Implants
Subperiosteal implants are placed beneath the gum tissue but above the jawbone, making them an option for patients with insufficient bone density who may not want to undergo bone grafting. This type of implant can be particularly advantageous for specialized dental practices focusing on patients with significant bone loss. While they offer stability without additional procedures, their invasive nature and higher costs may be a consideration for B2B buyers.
Zirconia Implants
Zirconia implants are crafted from a ceramic material known for its aesthetic qualities and biocompatibility. They are particularly appealing in cosmetic dentistry where the appearance of the implant is crucial. B2B buyers should weigh the benefits of aesthetic appeal against the limited long-term data available for zirconia implants. Additionally, they can be more expensive than traditional titanium implants, which may affect purchasing decisions.
One-Piece Implants
One-piece implants integrate both the post and abutment into a single unit, streamlining the surgical procedure and reducing treatment time. This design minimizes the risk of bacterial contamination, making it a popular choice for urgent care and fast-track dental clinics. However, they may not be suitable for all patients, particularly those requiring adjustments or replacements, which could be a significant factor for B2B buyers.
Mini Implants
Mini implants are characterized by their smaller diameter, making them ideal for narrow spaces or as a temporary solution. They are often used in orthodontics and for stabilizing immediate dentures. B2B buyers may find their minimally invasive nature and quick placement appealing, but should also consider their lower stability and unsuitability for heavy loads, which can limit their application in certain patient cases.
Related Video: Single Tooth Dental Implants: Everything You Need To Know
Key Industrial Applications of single tooth dental implants
| Industry/Sector | Specific Application of single tooth dental implants | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Dental Clinics | Restoration of single missing teeth | Enhanced patient satisfaction and retention | Compliance with local regulations; quality certifications |
| Dental Laboratories | Custom implant fabrication for individual patients | Improved precision and fit for patients | Material sourcing; advanced manufacturing capabilities |
| Medical Device Suppliers | Distribution of dental implant systems | Access to a growing market with high demand | Reliability of suppliers; regulatory approvals |
| Insurance Providers | Coverage for dental implant procedures | Increased client base and service offerings | Understanding of implant types; partnerships with clinics |
| Dental Education Centers | Training on single tooth implant procedures | Enhanced educational offerings; improved training outcomes | Accreditation of training programs; latest techniques and technology |
Dental Clinics
Single tooth dental implants are vital for dental clinics, providing an effective solution for patients with a single missing tooth. These implants restore both functionality and aesthetics, significantly enhancing patient satisfaction. Clinics must ensure compliance with local regulations and obtain quality certifications for the implants used. Additionally, establishing partnerships with reputable implant manufacturers can streamline the procurement process, ensuring access to high-quality products.
Dental Laboratories
In dental laboratories, single tooth dental implants are often custom fabricated to meet the unique anatomical requirements of individual patients. This application demands high precision to ensure a perfect fit, which can enhance the success rate of the implant procedure. Buyers in this sector should consider the sourcing of high-quality materials and the capabilities of manufacturers to produce customized solutions efficiently. Staying updated with advancements in dental technology will also be crucial.
Medical Device Suppliers
Medical device suppliers play a critical role in the distribution of single tooth dental implants to clinics and laboratories. This sector benefits from the rising demand for dental solutions, positioning suppliers as key facilitators in the market. When sourcing implants, suppliers must focus on the reliability of their manufacturers and ensure that all products have the necessary regulatory approvals. Establishing strong relationships with dental professionals can also enhance market penetration.
Insurance Providers
Insurance providers increasingly include coverage for single tooth implant procedures, recognizing the growing demand for dental restoration options. This application allows insurers to expand their client base and enhance their service offerings. Understanding the various types of implants and their associated costs is essential for insurance providers to create comprehensive plans. Building partnerships with dental clinics can facilitate smoother claims processes and improve customer satisfaction.
Dental Education Centers
Dental education centers utilize single tooth dental implants in their training programs to teach students the latest techniques in implantology. This application enhances the educational offerings and prepares future dental professionals for real-world scenarios. Buyers in this sector should focus on the accreditation of training programs and ensure that the latest techniques and technologies are incorporated into the curriculum. Collaborating with experienced practitioners can also provide valuable insights for students.
Related Video: How Dentists Insert Dental Implants
Strategic Material Selection Guide for single tooth dental implants
When selecting materials for single tooth dental implants, B2B buyers must consider a variety of factors that influence performance, durability, and compliance with international standards. Here, we analyze four common materials used in dental implants: titanium, zirconia, PEEK (Polyether ether ketone), and cobalt-chromium alloys. Each material has unique properties, advantages, and disadvantages that can impact their suitability for different applications.
Titanium
Key Properties:
Titanium is renowned for its biocompatibility, strength, and corrosion resistance. It can withstand high temperatures and pressures, making it ideal for dental applications. Its modulus of elasticity is similar to that of human bone, which helps in reducing stress shielding.
Pros & Cons:
The primary advantage of titanium is its durability and longevity, as it can resist wear and corrosion over time. However, titanium implants can be more expensive to manufacture and may require specialized equipment for machining. Additionally, while titanium is generally well-tolerated, some patients may experience allergic reactions.
Impact on Application:
Titanium is compatible with various media, including saliva and bone, making it a preferred choice for dental implants. Its excellent osseointegration properties ensure a strong bond with the jawbone, which is crucial for stability.
Considerations for International Buyers:
Buyers from regions like Africa and South America should be aware of the varying regulations regarding titanium implants. Compliance with standards such as ASTM F136 (for titanium alloys) is essential. Additionally, buyers should consider the availability of titanium implants in their local markets.
Zirconia
Key Properties:
Zirconia is a ceramic material that offers high strength and excellent aesthetics. It is highly resistant to wear and corrosion and has a low thermal conductivity, which is beneficial for patient comfort.
Pros & Cons:
The aesthetic appeal of zirconia makes it an attractive option for anterior implants, as it closely resembles natural tooth color. However, zirconia is more brittle than titanium, which can lead to fracture under excessive stress. The manufacturing process can also be complex and costly.
Impact on Application:
Zirconia implants are particularly suitable for patients seeking a natural look, especially in visible areas. However, their brittleness may limit their use in posterior applications where higher mechanical loads are expected.
Considerations for International Buyers:
Zirconia implants must meet specific standards, such as ISO 13356 for biocompatibility. Buyers should also consider the availability of zirconia implants in their regions and whether local dental practitioners are experienced in their placement.
PEEK (Polyether ether ketone)
Key Properties:
PEEK is a high-performance polymer known for its excellent mechanical properties and biocompatibility. It has a high resistance to temperature and chemicals, making it suitable for various dental applications.
Pros & Cons:
PEEK’s lightweight nature and flexibility can reduce stress on surrounding bone structures. However, it lacks the same level of osseointegration as titanium and zirconia, which may affect long-term stability. Additionally, PEEK implants can be less aesthetically pleasing compared to ceramic options.
Impact on Application:
PEEK is often used in cases where flexibility and reduced weight are essential, such as in patients with specific anatomical considerations. Its chemical resistance allows it to perform well in the oral environment.
Considerations for International Buyers:
Compliance with standards such as ISO 10993 for biocompatibility is crucial. Buyers should also be aware of the manufacturing capabilities in their regions, as PEEK implants may not be as widely available as titanium or zirconia options.
Cobalt-Chromium Alloys
Key Properties:
Cobalt-chromium alloys are known for their high strength, corrosion resistance, and wear resistance. They can withstand significant mechanical loads, making them suitable for dental applications.
Pros & Cons:
The primary advantage of cobalt-chromium alloys is their durability and resistance to deformation. However, they can be more challenging to manufacture and process compared to titanium. Additionally, there may be concerns regarding biocompatibility for some patients.
Impact on Application:
These alloys are particularly effective in load-bearing applications, such as posterior implants. Their corrosion resistance makes them suitable for long-term use in the oral environment.
Considerations for International Buyers:
Buyers must ensure compliance with relevant standards, such as ASTM F75 for cobalt-chromium alloys. Understanding local regulations and the availability of these materials is critical for successful procurement.
Summary Table
| Material | Typical Use Case for single tooth dental implants | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Titanium | Standard dental implants | Excellent durability and osseointegration | Potential for allergic reactions | High |
| Zirconia | Aesthetic anterior implants | Natural tooth-like appearance | Brittle and prone to fracture | Medium |
| PEEK | Flexible implant applications | Lightweight and flexible | Lower osseointegration | Medium |
| Cobalt-Chromium Alloys | Load-bearing posterior implants | High strength and corrosion resistance | Manufacturing complexity | High |
This strategic material selection guide provides B2B buyers with critical insights into the various materials used for single tooth dental implants. Understanding these factors will aid in making informed purchasing decisions that align with regional compliance and market demands.
In-depth Look: Manufacturing Processes and Quality Assurance for single tooth dental implants
Manufacturing Processes for Single Tooth Dental Implants
The manufacturing of single tooth dental implants involves a series of meticulously controlled processes that ensure the final product meets stringent quality and performance standards. For B2B buyers, understanding these processes is crucial to selecting a reliable supplier. Below are the main stages of manufacturing, along with key techniques used in the production of these dental implants.
Main Stages of Manufacturing
-
Material Preparation
– The choice of materials is critical. Titanium and zirconia are the most commonly used materials due to their biocompatibility and strength.
– Material Sourcing: Suppliers should ensure that materials are sourced from certified vendors who comply with international standards.
– Pre-Processing: Materials undergo initial processing such as cleaning, cutting, and surface treatment to remove impurities and enhance adhesion properties. -
Forming
– The forming stage involves shaping the implant. Techniques include:- CNC Machining: Computer Numerical Control (CNC) machining is widely used to achieve precise dimensions and tolerances.
- Additive Manufacturing: This method allows for complex geometries and can reduce material waste. It is particularly useful for creating intricate implant designs.
- The forming process must ensure that the implant’s surface roughness is optimized to promote osseointegration.
-
Assembly
– For single-piece implants, this stage is simpler as it involves no assembly of separate components. However, for some designs, the abutment and post may be fused.
– Sintering: In the case of zirconia implants, sintering is performed to enhance material properties and finalize the shape.
– Surface Treatments: Surface modifications like sandblasting or acid etching improve the implant’s surface characteristics, facilitating better integration with the bone. -
Finishing
– This stage includes polishing and coating processes to enhance aesthetic appeal and functionality.
– Quality Checks: Final checks are performed to ensure that the implants meet the required specifications before packaging.
Quality Assurance in Manufacturing
Quality assurance (QA) is paramount in the production of dental implants. It ensures that products are safe, effective, and compliant with international regulatory requirements.
Relevant International Standards
- ISO 9001: This standard specifies requirements for a quality management system (QMS) and is essential for ensuring consistent quality in manufacturing.
- ISO 13485: Specifically tailored for medical devices, this standard outlines the requirements for a comprehensive quality management system.
- CE Marking: For products sold in Europe, obtaining a CE mark signifies compliance with EU safety and performance standards.
- FDA Approval: In the United States, the FDA regulates dental implants as medical devices, and manufacturers must comply with specific premarket requirements.
Quality Control Checkpoints
-
Incoming Quality Control (IQC)
– Materials are inspected upon arrival to ensure they meet specified standards. This includes checking for material certifications and physical properties. -
In-Process Quality Control (IPQC)
– Continuous monitoring during the manufacturing process is critical. This includes regular checks of machine calibration and production parameters to ensure adherence to specifications. -
Final Quality Control (FQC)
– Once the manufacturing process is complete, a thorough inspection of the final product is conducted. This includes dimensional checks, surface integrity assessments, and functionality tests.
Common Testing Methods
- Mechanical Testing: Implants undergo various mechanical tests such as tensile, compression, and fatigue testing to ensure they can withstand the forces experienced in the mouth.
- Biocompatibility Testing: This is critical for ensuring that the materials used do not provoke an adverse reaction in the body.
- Sterilization Validation: Confirmation that the sterilization process is effective and that the implants are free of contaminants prior to packaging.
Verifying Supplier Quality Control
B2B buyers must take proactive steps to verify the quality control processes of potential suppliers. Here are effective strategies:
- Supplier Audits: Conduct regular audits to assess compliance with quality standards and manufacturing processes. This can include reviewing documentation and observing production practices.
- Quality Reports: Request comprehensive quality reports that detail testing results, process validations, and compliance with international standards.
- Third-Party Inspections: Engaging third-party inspection services can provide an unbiased assessment of the supplier’s manufacturing practices and quality control measures.
Quality Control and Certification Nuances for International Buyers
For international B2B buyers, particularly those from Africa, South America, the Middle East, and Europe, understanding regional regulations and certification nuances is essential:
- Regulatory Variations: Different regions have specific requirements for medical devices. For example, the FDA in the US, CE marking in Europe, and ANVISA in Brazil each have unique processes.
- Cultural and Market Considerations: Buyers should be aware of local market expectations regarding implant quality, material preferences, and pricing sensitivity.
- Local Partnerships: Establishing partnerships with local regulatory consultants can help navigate complex certification processes, ensuring compliance with both local and international standards.
Conclusion
Understanding the manufacturing processes and quality assurance measures for single tooth dental implants is vital for B2B buyers. By focusing on supplier qualifications, quality control practices, and adherence to international standards, buyers can ensure they procure high-quality implants that meet patient needs and regulatory requirements. This diligence not only enhances the safety and efficacy of dental implants but also builds trust and reliability in supplier relationships.
Related Video: Single Dental Implant Procedure Animated
Comprehensive Cost and Pricing Analysis for single tooth dental implants Sourcing
The cost structure for single tooth dental implants is multifaceted, encompassing various components that contribute to the overall pricing. Understanding these components is essential for international B2B buyers looking to optimize their sourcing strategies.
Cost Components
-
Materials: The primary materials used in dental implants typically include titanium and zirconia. Titanium is favored for its strength and biocompatibility, while zirconia offers aesthetic advantages. The choice of material significantly affects the cost, with titanium generally being less expensive than zirconia.
-
Labor: Labor costs vary based on the region and the expertise required for manufacturing. Skilled labor is essential for ensuring precision in implant production, which can lead to higher costs in regions with a limited labor pool.
-
Manufacturing Overhead: This encompasses the indirect costs associated with production, such as utilities, rent, and equipment maintenance. Manufacturers in regions with high operational costs may pass these expenses onto buyers.
-
Tooling: Specialized tooling for the manufacturing of implants can represent a significant upfront investment. The cost of tooling is often amortized over the production volume, affecting the unit price.
-
Quality Control (QC): Rigorous quality control is crucial in the dental industry to ensure safety and efficacy. The costs associated with QC processes, including testing and compliance with regulatory standards, can add to the overall price of implants.
-
Logistics: Shipping and handling costs, particularly for international transactions, can vary widely based on distance, shipping method, and customs regulations. Incoterms play a critical role in determining who bears these costs.
-
Margin: Manufacturers typically include a profit margin that reflects their operational risks and market positioning. This margin can vary based on the supplier’s reputation, market demand, and competition.
Price Influencers
Several factors can influence the final price of dental implants:
-
Volume/MOQ (Minimum Order Quantity): Bulk purchasing often leads to discounts. Buyers should negotiate for lower prices based on higher order volumes.
-
Specifications/Customization: Customized implants tailored to specific clinical needs may incur additional costs. Buyers should weigh the benefits of customization against the price increases.
-
Quality/Certifications: Implants that meet stringent regulatory standards (e.g., ISO 13485, CE marking) may command higher prices due to the associated compliance costs.
-
Supplier Factors: The reputation and reliability of suppliers can impact pricing. Established suppliers with a history of quality may charge a premium.
-
Incoterms: Understanding Incoterms is crucial for managing logistics costs. Terms like FOB (Free On Board) and CIF (Cost, Insurance, and Freight) can significantly influence total costs.
Buyer Tips
-
Negotiation: Engage in proactive negotiations with suppliers. Discuss volume discounts and payment terms to achieve cost savings.
-
Cost-Efficiency: Evaluate the total cost of ownership, not just the purchase price. Consider factors like longevity, maintenance, and potential complications related to the implants.
-
Pricing Nuances: Be aware that pricing can vary significantly between regions. For buyers from Africa, South America, the Middle East, and Europe, understanding local market dynamics is essential for competitive sourcing.
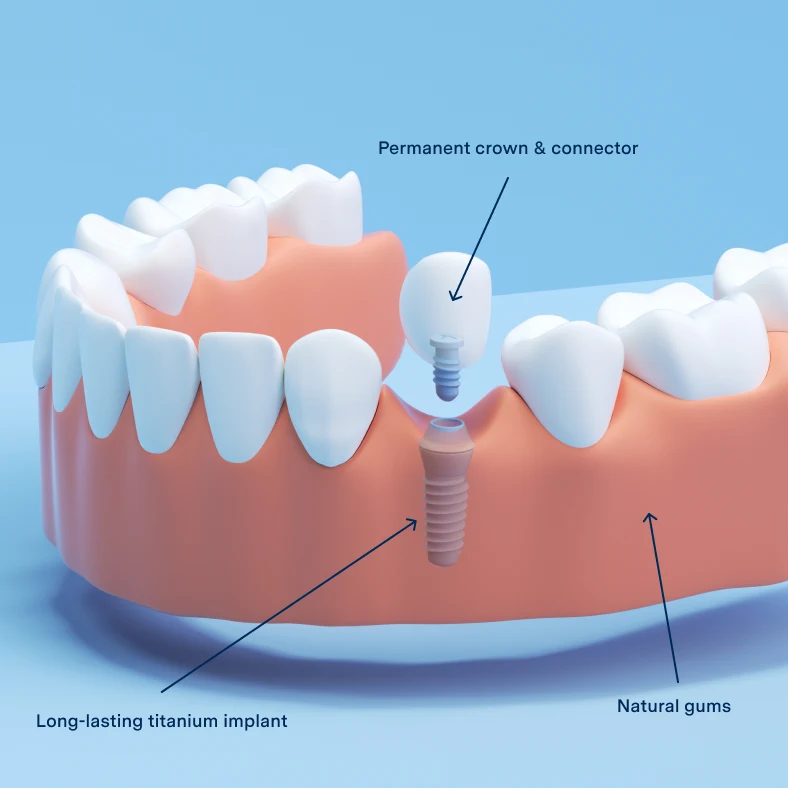
Illustrative Image (Source: Google Search)
-
Regulatory Compliance: Ensure that the implants meet the necessary regulatory standards in your target market. Compliance can affect both pricing and the ability to sell in certain regions.
-
Supplier Relationships: Build long-term relationships with suppliers to facilitate better pricing and terms over time. Loyalty can often lead to preferential treatment and discounts.
Disclaimer
Prices for single tooth dental implants can vary widely based on the factors outlined above. The figures provided are indicative and should be verified through direct supplier inquiries to obtain accurate pricing tailored to specific needs.
Spotlight on Potential single tooth dental implants Manufacturers and Suppliers
This section looks at several manufacturers active in the ‘single tooth dental implants’ market. This is a representative sample for illustrative purposes; B2B buyers must conduct extensive due diligence before any transaction. Information is synthesized from public sources and general industry knowledge.
Essential Technical Properties and Trade Terminology for single tooth dental implants
Understanding the critical technical properties and industry terminology related to single tooth dental implants is essential for international B2B buyers. This knowledge helps in making informed purchasing decisions, ensuring compliance with regulations, and optimizing supply chain management.
Key Technical Properties of Single Tooth Dental Implants
-
Material Grade
– Definition: Dental implants are commonly made from titanium or zirconia. Titanium, especially grade 5 (Ti-6Al-4V), is preferred for its strength and biocompatibility.
– Importance: The material grade affects the implant’s durability and the body’s acceptance of the implant. Understanding material specifications helps buyers select implants that meet their market’s regulatory and performance standards. -
Surface Treatment
– Definition: The surface of dental implants can be treated to enhance osseointegration, such as through sandblasting or acid-etching.
– Importance: The surface treatment influences the implant’s ability to bond with bone. For B2B buyers, selecting implants with optimal surface characteristics can lead to better patient outcomes and satisfaction. -
Length and Diameter
– Definition: Implants come in various lengths (usually between 8 mm to 15 mm) and diameters (typically 3.3 mm to 5 mm).
– Importance: The correct size is crucial for successful implantation in different anatomical situations. Buyers must understand their target demographics and common anatomical challenges in their regions to choose appropriate sizes. -
Tolerances
– Definition: Tolerances refer to the allowable variations in dimensions during manufacturing, ensuring that components fit together correctly.
– Importance: Tight tolerances lead to better fit and stability. B2B buyers should prioritize suppliers with stringent quality control to minimize complications during implantation. -
Mechanical Properties
– Definition: This includes tensile strength, shear strength, and fatigue resistance, which indicate how well the implant can withstand forces during chewing.
– Importance: Understanding these properties helps buyers assess the long-term performance and reliability of implants, which is critical for maintaining a good reputation and reducing warranty claims.
Common Trade Terminology in Dental Implants
-
OEM (Original Equipment Manufacturer)
– Definition: A company that produces parts or equipment that may be marketed by another manufacturer.
– Importance: Understanding OEM relationships is vital for buyers who are looking to source implants or components that meet specific brand requirements or quality standards. -
MOQ (Minimum Order Quantity)
– Definition: The smallest quantity of a product that a supplier is willing to sell.
– Importance: Knowing the MOQ helps buyers assess the feasibility of sourcing implants based on their inventory needs and financial constraints. -
RFQ (Request for Quotation)
– Definition: A document issued by a buyer to solicit quotes from suppliers for specific products or services.
– Importance: An RFQ process allows buyers to compare prices and terms from different suppliers, ensuring they get the best deal while meeting quality standards. -
Incoterms (International Commercial Terms)
– Definition: A series of pre-defined commercial terms published by the International Chamber of Commerce (ICC) relating to international commercial law.
– Importance: Familiarity with Incoterms is essential for understanding shipping responsibilities, costs, and risks associated with international trade, helping buyers avoid unexpected expenses. -
CE Marking
– Definition: A certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area.
– Importance: For buyers in Europe, ensuring that dental implants have CE marking is crucial for compliance with regulatory requirements, impacting market access.

Illustrative Image (Source: Google Search)
- Sterilization Validation
– Definition: The process of proving that a sterilization method effectively eliminates all microbial life.
– Importance: Buyers must ensure that the implants they purchase are validated for sterilization to maintain patient safety and adhere to health regulations.
By understanding these technical specifications and trade terms, international B2B buyers can make informed decisions that align with their business goals and regulatory requirements.
Navigating Market Dynamics, Sourcing Trends, and Sustainability in the single tooth dental implants Sector
Market Overview & Key Trends
The global market for single tooth dental implants is experiencing robust growth, driven by an aging population, increasing awareness of oral health, and advancements in dental technology. International B2B buyers, particularly from Africa, South America, the Middle East, and Europe, should note several key trends that are shaping this sector.
-
Technological Advancements: Innovations in materials and manufacturing processes, such as the use of 3D printing and computer-aided design (CAD), have enhanced the precision and customization of dental implants. This trend allows for faster production times and tailored solutions that meet specific patient needs.
-
Growing Demand for Minimally Invasive Procedures: There is a marked shift towards less invasive techniques, such as flapless implant surgery, which reduces recovery time and improves patient satisfaction. This trend is particularly relevant for markets where patient comfort and quick turnaround are priorities.
-
Cost Competitiveness: As competition increases, manufacturers are focusing on cost-effective solutions without compromising quality. Buyers from emerging markets are particularly sensitive to price, making it essential for suppliers to balance affordability with high standards of dental care.
-
Regulatory Compliance: Understanding the regulatory landscape is crucial for B2B buyers. Different regions have varying requirements for product certifications, such as CE marking in Europe and FDA approvals in the U.S. Buyers must ensure that their suppliers comply with these regulations to avoid potential disruptions.
These trends present opportunities for B2B buyers to leverage technological advancements, optimize costs, and ensure compliance while meeting the evolving needs of dental professionals and patients alike.
Sustainability & Ethical Sourcing in B2B
As sustainability becomes a paramount concern across industries, the dental implant sector is not exempt. B2B buyers should prioritize sourcing from suppliers who demonstrate a commitment to environmental responsibility and ethical practices.
-
Environmental Impact: The production of dental implants involves significant resource consumption and waste generation. Buyers should seek manufacturers that utilize sustainable practices, such as reducing waste, recycling materials, and minimizing energy consumption in their production processes.
-
Ethical Supply Chains: Ensuring that the materials used in dental implants are sourced ethically is critical. This includes verifying that titanium and zirconia are obtained from suppliers who adhere to fair labor practices and environmental standards. Buyers can enhance their corporate social responsibility (CSR) profile by opting for suppliers that are transparent about their sourcing practices.
-
Green Certifications: Look for manufacturers that possess certifications such as ISO 14001 (Environmental Management) or those that utilize biocompatible materials that are less harmful to the environment. These certifications serve as indicators of a commitment to sustainability and can help differentiate suppliers in a competitive market.
Incorporating sustainability into sourcing strategies not only mitigates environmental impact but also aligns with the growing consumer preference for responsible and ethical business practices.
Brief Evolution/History
The development of single tooth dental implants has evolved significantly over the past few decades. Initially, implants were rudimentary and often unsuccessful, leading to high failure rates. However, the introduction of titanium in the 1960s revolutionized the field due to its biocompatibility and strength, paving the way for more successful implant procedures.
In the late 20th century, advancements in implant design and surgical techniques further improved outcomes. The emergence of digital technologies, such as CAD/CAM systems, has enabled more precise implant placement and customization, thereby enhancing patient care. As the market continues to grow, ongoing innovations are likely to shape the future of single tooth dental implants, making them more accessible and effective for patients worldwide. For B2B buyers, understanding this evolution is essential for making informed sourcing decisions in a rapidly changing landscape.
Frequently Asked Questions (FAQs) for B2B Buyers of single tooth dental implants
-
What criteria should I consider when vetting suppliers of single tooth dental implants?
When vetting suppliers, prioritize their certifications and compliance with international standards such as ISO 13485 for medical devices and CE marking for products sold in Europe. Investigate their manufacturing processes, quality control measures, and track record in the industry. Request references from other B2B partners and assess their responsiveness and customer service. Additionally, consider their experience with international shipping and handling customs regulations, especially if you’re sourcing from different continents. -
Can I customize single tooth dental implants to meet specific patient needs?
Yes, many suppliers offer customization options for single tooth dental implants. This can include variations in material (like titanium vs. zirconia), implant size, and design features tailored to specific clinical requirements. When discussing customization, ensure that the supplier can provide detailed documentation and compliance with medical device regulations in your target market. Engage in early communication to clarify your needs and confirm lead times for customized orders. -
What are the minimum order quantities (MOQ) and lead times for single tooth dental implants?
MOQs can vary significantly between suppliers, often influenced by the type of implant and customization options. Generally, suppliers may require a MOQ ranging from 10 to 100 units per order. Lead times also depend on the supplier’s production capacity and the complexity of the order, typically ranging from 2 to 8 weeks. Always confirm these details upfront to plan your inventory effectively and avoid potential disruptions in your supply chain. -
What payment terms are typically offered for international orders of dental implants?
Payment terms can vary by supplier, but common arrangements include upfront payment, partial payment before shipment, or net terms (e.g., net 30, net 60). Be cautious about suppliers that demand full payment upfront without a clear return policy or warranty. Consider using secure payment methods, such as letters of credit or escrow services, which can provide additional protection against disputes. Always clarify terms in writing to avoid misunderstandings. -
How can I ensure quality assurance and certification compliance for dental implants?
Request copies of the supplier’s quality management system certifications and product testing results. Verify that the implants comply with relevant international regulations such as the FDA in the U.S., CE marking in Europe, and local regulations in your region. Conduct audits or inspections if possible, and consider third-party quality assurance services to validate the supplier’s claims. Documentation should include certificates of conformity, safety data sheets, and any clinical evidence supporting the product’s efficacy. -
What logistics considerations should I be aware of when importing dental implants?
Logistics can pose challenges, including customs clearance, shipping costs, and potential tariffs. Ensure that the supplier has experience with international shipping and can provide necessary documentation for customs, such as commercial invoices and certificates of origin. Collaborate with a reliable logistics partner who understands the medical device sector and can navigate regulatory requirements in your country. Also, account for potential delays and plan your inventory levels accordingly. -
What steps should I take if I encounter a dispute with my supplier?
In the event of a dispute, begin by reviewing the contract and any agreements made regarding terms, quality expectations, and delivery schedules. Open a line of communication with your supplier to discuss the issue and seek resolution amicably. If the dispute cannot be resolved directly, consider mediation or arbitration as outlined in your contract. Document all communications and maintain a clear record of the issue to support your case should legal action become necessary. -
What are the common challenges faced when sourcing dental implants internationally?
Common challenges include regulatory compliance across different markets, variations in product quality, and differing standards of care. Language barriers can complicate communication, and cultural differences may influence business practices. Additionally, logistics and shipping delays can impact delivery timelines. To mitigate these challenges, conduct thorough market research, build strong relationships with suppliers, and consider partnering with local distributors who understand the nuances of the market in your region.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Strategic Sourcing Conclusion and Outlook for single tooth dental implants
The landscape of single tooth dental implants presents a wealth of opportunities for international B2B buyers, particularly in Africa, South America, the Middle East, and Europe. Strategic sourcing is essential in navigating this market, enabling buyers to secure high-quality implants while optimizing costs and compliance with regional regulations.
Key Takeaways:
- Quality Assurance: Prioritize suppliers with robust quality management systems, such as ISO 13485 certification, to ensure product reliability and patient safety.
- Regulatory Compliance: Understand the specific regulatory requirements in your region, including CE marking in Europe and FDA approval in the U.S., to mitigate risks associated with non-compliance.
- Cost-Effectiveness: Evaluate one-piece implants for their potential cost savings, reduced treatment times, and lower risk of complications, which can enhance patient satisfaction and loyalty.
As the demand for dental implants continues to rise, international buyers should leverage strategic sourcing to build strong partnerships with manufacturers who demonstrate innovation and reliability. Looking ahead, consider investing in advanced technologies and sustainable practices within your supply chain to enhance your competitive edge and meet the evolving needs of your market.
